Table 1.
Cyclization Reactions to Form 22
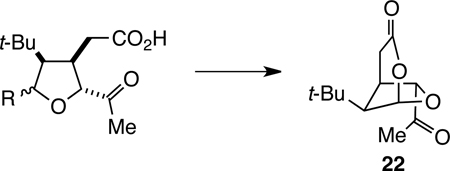 | |||
|---|---|---|---|
| Entry | R | Conditions | Yield 22a |
| 1 | OH (35) | DEAD, PPh3, CH2Cl2 | 0% |
| 2 | OH (35) | 0.2 equiv CSA, CHCl3, rt, 12 h | 54% |
| 3 | OMe (37) | 1.2 equiv BF3• OEt2, CH2Cl2, 0 °C, 1 h | 66% |
| 4 | F (39) | 2 equiv SnCl2, DMF, rt, 18 h | 71% |
Yield reported as conversion from methyl ester precursor (34, 36, and 38, respectively)
