Fig. 4.
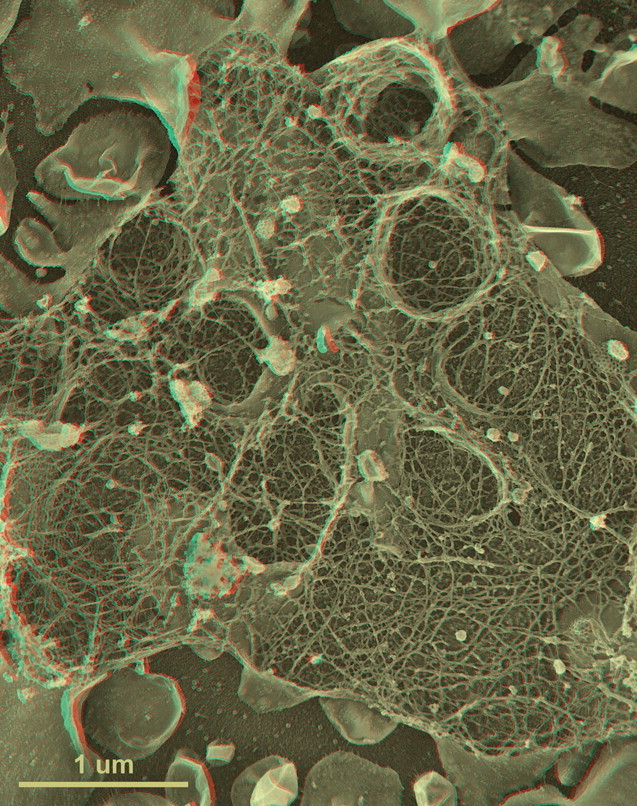
First-time published image of a brand new variation of the QF-DE-RR procedure for imaging the interiors of unroofed cells, which involves doing everything on a pre-formed carbon substrate rather than on glass, so that the platinum replica does not ever need to be separated from its substrate before TEM viewing. This eliminates the huge problem of replica breakage that has always plagued the field – the breakage occurring when the replica is separated from the underlying cells and substrate, in order to be viewed in the TEM. Also, because freeze-fracture replicas were always so fragile, they formerly had to be supported by carbon films deposited on top of them, which tended to confuse or obscure the final images. Now, with the carbon substrate underneath the cells, this ‘backing’ or support film can be eliminated, and vastly larger and more stable replicas can be obtained, even so. This permits approaches possible never before, as in this experiment, where HeLa cells that were in suspension culture, and thus were highly ‘blebby’, were exposed to glow-discharged carbon and allowed to attach to it for only 1 min before they were ‘unroofed’, thereby yielding images of the inside surface of such highly dynamic cell processes as blebs (the obvious, micron-sized circular domains in the cell) where actin polymerization is known to be going on actively [168,169] but could never before be visualized at this resolution. Close inspection of the circular domains illustrates that actin polymerization in situ is via the formation of y-shaped branches from the existing actin filaments, exactly as was predicted from the ‘dendritic branching’ model we derived earlier from our in vitro imaging of actin and Arp2/3 [170].
