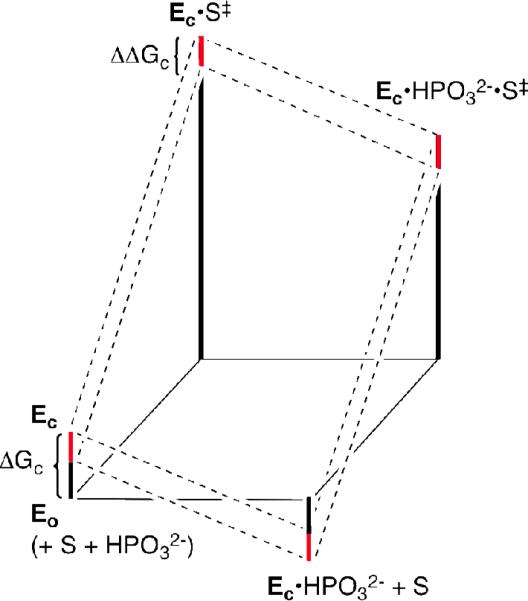
Figure 3.
Proposed free energy profiles for the turnover of glycolaldehyde (S) by free TIM (Eo) and by the phosphite-liganded enzyme Ec•HPO32-. The red bars show the effect of the L232A mutation on the barrier for the conformational change from Eo to Ec (ΔΔGc). The effect of this change in ΔGc on turnover of the substrate pieces is shown by a comparison of the reaction profiles for wildtype TIM (upper dashed lines) and L232A mutant TIM (lower dashed lines).