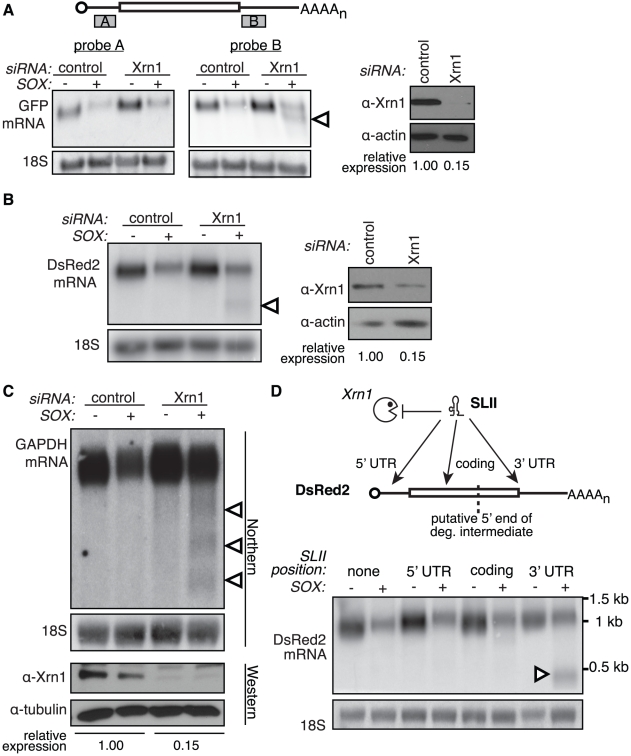
Figure 1. Xrn1 is required for the removal of a 3′ intermediate during SOX-mediated mRNA degradation.
(A–B) 293T cells were treated with control or Xrn1 siRNAs, then transfected with the indicated reporters +/− SOX. The level of Xrn1 protein depletion is shown on the right, with actin as a loading control. (A) Diagram depicts location of probes on the GFP message. Northern blots using probes against either the 5′ (probe A; left panel) or 3′ (probe B; right panel) UTR of the GFP mRNA reporter or 18S. (B) Northern blots using probes against the 3′ UTR of the DsRed2 mRNA reporter, or 18S (left panels). (C) 293T cells were treated with control or Xrn1 shRNAs, then transfected with GFP (−) or GFP-SOX (+). Cells were then selected for GFP fluorescence by FACS before RNA and protein collection. RNA was Northern blotted with probes to the 3′ end of the GAPDH coding region, or to 18S. The level of Xrn1 knockdown was assessed by Western blot, using tubulin as a loading control. In panels A, B and C, arrowheads denote degradation intermediates. (D) A flaviviral Xrn1-blocking element (SLII) was inserted at different positions within the DsRed2 mRNA. RNA from 293T cells transfected with the indicated reporter +/− SOX was Northern blotted using a probe directed against the 3′ UTR of the DsRed2 reporter, or 18S. The full-length mRNA is ∼1.2 kb, and the expected size of the fragment protected from Xrn1 degradation by SLII within the 5′ UTR is ∼1.1 kb, within the coding region is ∼900 bp, and within the 3′ UTR is ∼500 bp. Arrowhead denotes protected fragment.