Abstract
Glucosamine, a naturally occurring amino monosaccharide, has been reported to play a role in the regulation of apoptosis more than half century. However the effect of glucosamine on tumor cells and the involved molecular mechanisms have not been thoroughly investigated. Glucosamine enters the hexosamine biosynthetic pathway (HBP) downstream of the rate-limiting step catalyzed by the GFAT (glutamine:fluctose-6-phosphate amidotransferase), providing UDP-GlcNAc substrates for O-linked β-N-acetylglucosamine (O-GlcNAc) protein modification. Considering that O-GlcNAc modification of proteasome subunits inhibits its activity, we examined whether glucosamine induces growth inhibition via affecting proteasomal activity. In the present study, we found glucosamine inhibited proteasomal activity and the proliferation of ALVA41 prostate cancer cells. The inhibition of proteasomal activity results in the accumulation of ubiquitinated proteins, followed by induction of apoptosis. In addition, we demonstrated that glucosamine downregulated proteasome activator PA28γ and overexpression of PA28γ rescued the proteasomal activity and growth inhibition mediated by glucosamine. We further demonstrated that inhibition of O-GlcNAc abrogated PA28γ suppression induced by glucosamine. These findings suggest that glucosamine may inhibit growth of ALVA41 cancer cells through downregulation of PA28γ and inhibition of proteasomal activity via O-GlcNAc modification.
Keywords: apoptosis, glucosamine, glycosylation, Ki antigen, prostatic neoplasms, proteasome
Introduction
Glucosamine, a naturally occurring amino monosaccharide, is present in connective and cartilage tissues, and contributes to maintaining the strength, flexibility and elasticity of these tissues. Thus, glucosamine has been widely used to treat osteoarthritis in humans (Reginster et al., 2001). In addition to its chondroprotective action, glucosamine has been supposed to exert anticancer action more than five decades (Marlow and Bartlett, 1953; Quastel and Cantero, 1953; Fjelde et al., 1956; Sorkin and Fjelde, 1956; Ball et al., 1957; Luhrs, 1957; Kizer and Mc, 1959).
Between 2 and 5% of the glucose transported into cells is converted to UDP-GlcNAc (the donor sugar for the biosynthesis of O-GlcNAc) through the hexosamine biosynthetic pathway (HBP) (McClain, 2002). Elevated extracellular glucose or glucosamine concentrations lead to increased modification of intracellular proteins with O-GlcNAc (Thomas et al., 2003). The addition of O-GlcNAc to serine and threonine residues is a posttranslational modification of cytoplasmic and nuclear proteins that is thought to act in a manner analogous to protein phosphorylation. Changes in O-GlcNAc levels have been shown to alter the behavior of specific proteins by modulating enzyme activity, protein-protein interactions, DNA binding, subcellular localization, the half-life and proteolytic processing of proteins (Zachara et al., 2004). Therefore, treatment with glucosamine has also been widely used as a tool to investigate the effects of increased HBP flux on a variety of cell signaling pathways.
In this context, proteasome function has been shown to be regulated by the posttranslational modification (PTM) of its cap by O-GlcNAc (Han and Kudlow, 1997; Lee et al., 1999; Su et al., 2000; Zhang et al., 2003; Liu et al., 2004). Proteasomes are large complexes that carry out crucial roles in many cellular pathways by degrading proteins in the cytosol and nucleus of eukaryotic cells to enforce quality control and to regulate many cellular processes such as cell survival, proliferation, apoptosis and other critical cellular functions (Adams, 2004). Many proteins involved in cancer cell growth and survival are regulated by proteasomal degradation, making it an attractive therapeutic target (Adams, 2004).
Although the anticancer effect of glucosamine has been reported more than half century, the mechanism underlying its action remains poorly defined. Here for the first time we show that glucosamine reduced proteasome activity and induced growth arrest and apoptosis in ALVA41 prostate cancer cells. The decrease in proteasome activity occurred prior to apoptosis. We also found that glucosamine specifically downregulated PA28γ, one of the proteasome activator. Importantly, overexpression of PA28γ rescued the proteasome inhibition and apoptosis induced by glucosamine. In addition, we found inhibition of O-GlcNAc by alloxan or specific siRNA targeting of the key HBP enzyme UDP-N-acetylglucosamine (GlcNAc): polypeptide-)-β-acetylglucosaminyltransferase (OGT) blocked glucosamine induced suppression of PA28γ. Collectively, the current study suggested that glucosamine is likely to induce cell death in ALVA41 cells, possibly by affecting PA28γ expression and proteasome activity via O-GlcNAc modification.
Results
Glucosamine induces cell death in human ALVA41 prostate cancer cells
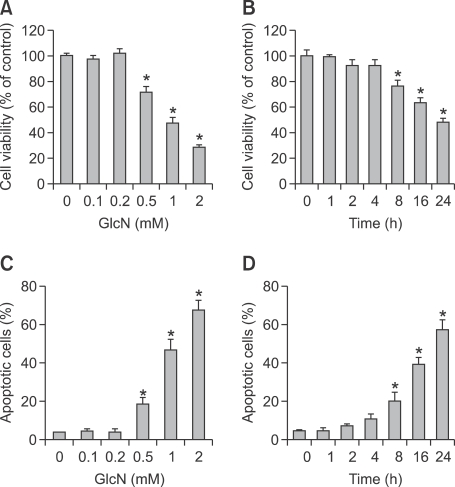
The effect of glucosamine on ALVA41 prostate cell proliferation was evaluated by MTT assay. Treatment with glucosamine potently decreased proliferation in ALVA41 cells in a dose-dependent manner (Figure 1A). The inhibitory effect was evident when ALVA41 cells were treated with 0.5-2 mM of glucosamine (Figure 1A). ALVA41 cells were then treated with 1mM of glucosamine for the indicated times. The cell viability was decreased after 8 h treatment, which was further increased afterwards (Figure 1B). To examine whether glucosamine exerts the proliferation inhibitory effect against ALVA41 cells via apoptosis induction, annexin V/PI double staining and subsequent flow cytometry was performed, which revealed an increase in the proportion of apoptotic cells compared with vehicle-treated in a dose-dependent manner (Figure 1C). Time course confirmed that 1mM of glucosamine caused significant apoptosis of ALVA41 cells at 8 h, which was further increased afterwards (Figure 1D).
Figure 1.
Glucosamine induces growth inhibition and apoptosis in ALVA41 cells. (A) ALVA41 cells were treated with various concentrations of glucosamine (GlcN) for 24 h, and cell viability was analyzed using MTT assay. (B) ALVA41 cells were treated with 1 mM of glucosamine for the indicated period and cell viability was analyzed using MTT assay. (C) ALVA41 cells were treated with various concentrations of glucosamine for 24 h, and apoptotic cells were analyzed using annexin V-FITC/propidium iodide (PI) double staining and subsequent flow cytometry. (D) ALVA41 cells were treated with 1 mM of glucosamine for the indicated period, and apoptotic cells were analyzed using annexin V-FITC/PI double staining and subsequent flow cytometry. *P < 0.01.
Glucosamine causes proteasome inhibition in human ALVA41 prostate cancer cells
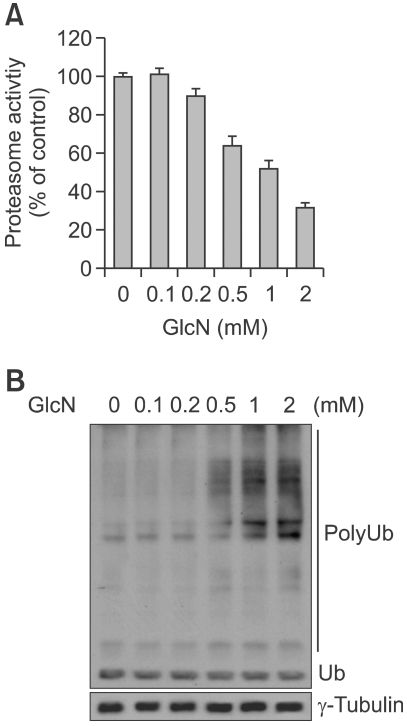
Cellular glucosamine treatment provides obligate substrates for O-GlcNAc modification of proteins, furthermore, the posttranslational modification of the mammalian proteasome by O-GlcNAc can inhibit its proteolytic function (Zhang et al., 2003, 2007). To verify whether glucosamine can affect the cellular 26S proteasome activity of ALVA41 cells, cells were treated with the indicated concentrations of glucosamine for 8 h, followed by the measurement of proteasome activity in the cell lysates prepared. The proteasomal chymotrypsin-like activity was inhibited by glucosamine in a dose-dependent manner (Figure 2A). Glucosamine caused approximately 10, 37, 49 or 70% inhibition at 0.2, 0.5, 1 or 2 mM, respectively. In accordance with inhibition of proteasomal activity, ubiquitinated proteins, which were tagged by polyubiquitins for the proteasome degradation, were accumulated in a dose-dependent manner; slight accumulation by 0.5 mM glucosamine treatment and further accumulation by 1-2 mM glucosamine (Figure 2B).
Figure 2.
Glucosamine reduces the proteasomal activity. (A) ALVA41 cells were treated with various concentrations of glucosamine for 8 h and the chymotrypsine-like activity of proteasomes was analyzed. (B) ALVA41 cells were treated as A, and polyubiquitinated proteins were analyzed using Western blot analysis.
Glucosamine-induced proteasome inhibition occurs prior to tumor cell death
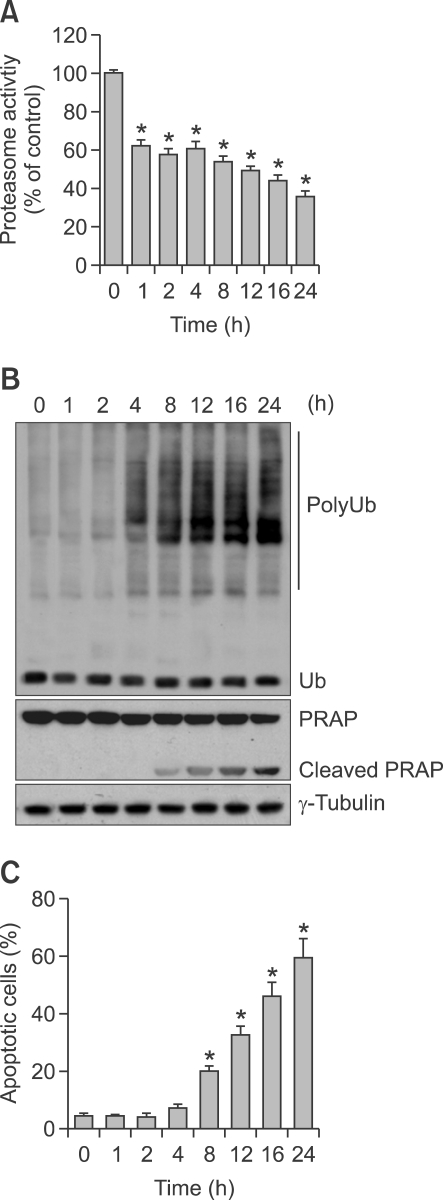
We performed kinetic experiments using ALVA41 cell lines to determine which event occurs first, proteasome inhibition or cell death induction. ALVA41 cells were treated with 1 mM of glucosamine for up to 24 h, followed by Western blotting and flow cytometry analysis. The proteasomal chymotrypsin-like activity in ALVA41 was inhibited around 40% at as early as 1 h after addition of glucosamine, which was lasted to 4 h and then further increased to 65% inhibition at 24 h (Figure 3A). Consistently, accumulation of ubiquitinated proteins was detected from 4 h to 24 h, peaked at 12-16 h during the treatment of glucosamine (Figure 3B). In a sharp contrast to the proteasome inhibition at early hours, cell death occurred in later hours. PARP cleavage, an indicator apoptotic cell death, was detected only after 8 h treatment of glucosamine (Figure 3B). We also performed annexin V/PI double staining and subsequent flow cytometry to measure the apoptotic cells in the cells after glucosamine treatment. Compared with untreated control, apoptotic cells were increased after 8 h and further increased afterwards (Figure 3C).
Figure 3.
Proteasome inhibition mediated by glucosamine occurs prior to induction of apoptosis. (A) ALVA41 cells were treated with 1 mM of glucosamine for the indicated period and chymotrypsine-like activity of proteasomes was analyzed. (B) Cells were treated as A, and Western blot analysis was performed using the indicated antibodies. (C) Cells were treated as A, and apoptotic cells were measured using annexin V/PI double staining and subsequent flow cytometry. *P < 0.01.
Overexpression of PA28γ rescues the inhibition of proteasomal activity as well as cell death induced by glucosamine
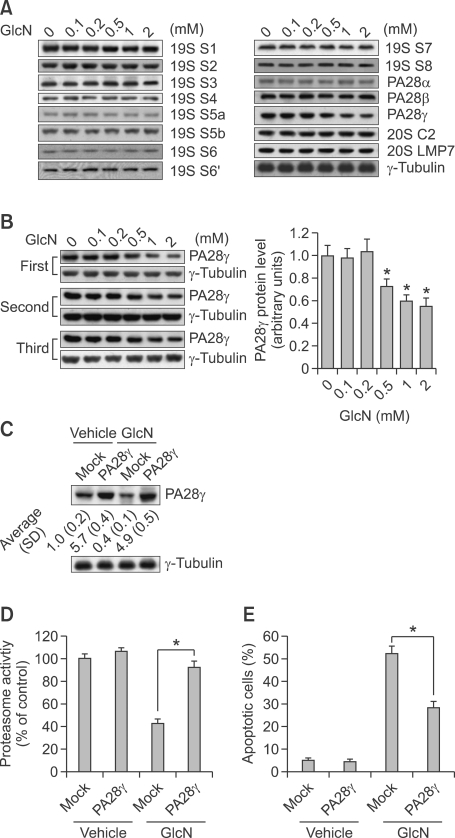
To investigate the potential mechanism(s) underlying inhibition of proteasomal activity by glucosamine, we analyzed the levels of some components of proteasome using Western blot analysis. Glucosamine markedly decreased the level of proteasome activator PA28γ, also known as 11S regulator (11S REGγ), whereas had little effect on proteasome activator PA28α, PA28β or other components (Figure 4A). The reduction of PA28γ by glucosamine was further confirmed by three different experiments (Figure 4B). To investigate the potential involvement of PA28γ in the inhibition of proteasome activity and growth mediated by glucosamine, we overexpressed PA28γ in ALVA41 cells (Figure 4C). Overexpression of PA28γ rescued glucosamine-induced downregulation of PA28γ (Figure 4C) and suppression of proteasomal activity (Figure 4D). Importantly, overexpression of PA28γ also markedly blocked cell apoptosis mediated by glucosamine (Figure 4E).
Figure 4.
Overexpression of PA28γ abrogates glucosamine-mediated proteasomal inhibition and apoptosis. (A) ALVA41 cells were treated with various concentrations of glucosamine for 24 h and Western blot analysis was performed using the indicated antibodies. (B) ALVA41 cells were treated with vehicle or the indicated concentrations of glucosamine for 24 h and Western blot analysis was performed to measure the expression level of PA28γ. The ratios vs. that of control (normalized by γ-tubulin) from the three different experiments were graphed. (C) 24 h after transfection with mock or PA28γ expression vector, ALVA41 cells were treated with vehicle or 1 mM of glucosamine for additional 24 h and PA28γ expression was analyzed using Western blot analysis. A representative image was presented, and the ratios vs. that of control (normalized by γ-tubulin) were noted at the bottom of the image (n = 3). (D) 24 h after transfection with mock or PA28γ expression vector, ALVA41 cells were treated with vehicle or 1 mM of glucosamine for additional 24 h and chymotrypsin-like proteasomal activity was measured. (E) 24 h after transfection with mock or PA28γ expression vector, ALVA41 cells were treated with vehicle or 1 mM of glucosamine for additional 24 h and apoptotic cells were measured using annexin V/PI double staining and subsequent flow cytometry. *P < 0.01.
O-GlcNAc modification is implicated in glucosamine-induced downregulation of PA28γ and proteasome inhibition
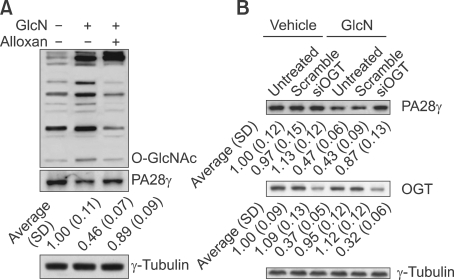
Glucosamine enters the HBP downstream of the rate-limiting step to provide UDP-GlcNAc for O-GlcNAc modification of proteins. Thus, we investigated the relationship between the glucosamine-induced O-GlcNAc modification and suppression of PA28γ expression by using alloxan, an inhibitor of O-GlcNAc modification. We confirmed that glucosamine increased protein O-GlcNAc modification, and alloxan effectively prevented the glucosamine-induced O-GlcNAc modification (Figure 5A). Of importance, alloxan abrogated the glucosamine-induced suppression of PA28γ (Figure 5A). Since chemical inhibitor may have limitations, potentially causing other "off target" effects on the cells, siRNA specific against OGT (siOGT) was utilized to bolster these data. Western blot analyses confirmed a decrease in OGT protein expression in siOGT transfected cells (Figure 5B). siOGT blocked glucosamine-mediated downregulation of PA28γ, while the scramble siRNA had no obvious effect (Figure 5B).
Figure 5.
O-GlcNAc modification is implicated in suppression of PA28γ by glucosamine. (A) ALVA41 cells were treated with 1 mM of glucosamine alone or combination with 0.5 mM of alloxan for 24 h and Western blot analysis was performed using the indicated antibodies. A representative image was presented, and the ratios vs. that of control (normalized by γ-tubulin) were noted at the bottom of the image (n = 3). (B) ALVA41 cells were transfected with scramble or specific siRNA against OGT for 24 h, then treated with vehicle or 1mM of glucosamine for additional 24 h and Western blot analysis was performed. A representative image was presented, and the ratios vs. that of control (normalized by-tubulin) were noted at the bottom of the image (n = 3).
Discussion
Glucosamine is widely used to relieve symptoms of osteoarthritis (Biggee and McAlindon, 2004), and its clinical safety and effects have been thoroughly evaluated (Anderson et al., 2005). Glucosamine enters the hexosamine biosynthetic pathway (HBP) downstream of the rate-limiting step catalyzed by the glutamine: fluctose-6-phosphate amidotransferase (GFAT). One consequence of increased HBP activity is an accumulation of UDP-GlcNAc (Marshall et al., 2004), which is the obligate substrate for the O-GlcNAc transferase (OGT), the enzyme responsible for O-linked β-N-acetylglucosamien (O-GlcNAc) protein modification (Dong and Hart, 1994). It is now recognized that the addition of O-GlcNAc to target proteins may modulate cellular functions, such as nuclear transport, transcription, translation, proteolytic degradation, cell signaling, apoptosis and cell shape (Thomas et al., 2003; Hart et al., 2007; Lingbeck et al., 2008). Protein glycosylation with O-GlcNAc is a reversible post-translational modification at serine and threonine residues on myriad nuclear and cytoplasmic proteins, which occurs with similar time scales, dynamics and stoichiometry as protein phosphorylation and interplays with phosphorylation in a "Yin Yang" manner (Hart et al., 2007). Phosphorylation of PA28γ by MEKK3 has been reported to increase the protein levels most likely by increasing its stability (Hagemann et al., 2003). In addition, using artificial neural network based PTM prediction methods (Kaleem et al., 2009), we found one in silico predicted Yin Yang site (Ser 248) and one false negative Yin Yang site (Ser 247). In this context, the current study demonstrated that glucosamine can induce the O-GlcNAc modification and inhibit PA28γ expression in ALVA41. Furthermore, alloxan, an OGT inhibitor eliminated the inhibitory effect of glucosamine on PA28γ expression. These findings suggest that possible involvement of glucosamine-induced O-GlcNAc modification of PA28γ in reduction of its level, which is under investigation in our lab.
PA28γ is a member of the PA28 family of proteins, which have been shown to bind specifically to 20S proteasomes and stimulate the hydrolysis of peptides. There are three PA28 homologs, called PA28α, β and γ. Interestingly two other members of the PA28 family of proteins, PA28α and PA28β, were not altered by glucosamine treatment, underlying the specificity of the reported downregulation of PA28γ by glucosamine in ALVA41 cells. In the current study, we found that PA28γ downregulation was implicated in proteasomal inhibition and cell death induced by glucosamine. Consist with our finding, several studies have reported a potential role of PA28γ in G1 cell cycle arrest and induction of apoptosis, indicating that PA28γ is an anti-apoptotic factor (Murata et al., 1999; Hagemann et al., 2003; Masson et al., 2005; Rechsteiner and Hill, 2005; Qian et al., 2007). In addition, abnormally high expression of PA28γ has been reported in some malignant cells (Roessler et al., 2006; Tagawa et al., 2008). The proposed anti-apoptotic properties of PA28γ could explain its downregulation in ALVA41 cell death induced by glucosamine.
In summary, the current study suggested a novel mechanism of action by which glucosamine could induce ALVA41 cell death by suppression of proteasomal activity, which is likely ascribed to reduction of proteasome activator PA28γ via O-GlcNAc modification.
Methods
Cell viability assays
For cell viability assay, cells were plated in 96-well dishes (1×104 cells/per well) and, the next day, were treated with or without apoptosis-inducing agents in 2% FBS containing media and grown over a 24 h period. Cell viability was assessed using the MTT assay (Chemicon, Bedford, MA) according to the manufacturer's instruction.
20S proteasome activity assay
Cell lysates (without protease inhibitors) were used to measure proteasome activity using 20S proteasome assay kit (Chemicon International, Temecula, CA) following the manufacturer's instructions. The assay is based on detection of the fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate LLVY-AMC. Levels of released AMC were measured using an excitation wavelength of 380 nm and an emission wavelength of 460 nm with an automatic multi-well plate reader. The relative activity was standardized by protein concentration, determined using Coomassie Protein Assay Reagent (Pierce, Rockford, IL).
Detection of apoptotic cell death
For apoptotic cell death assays, cells were washed twice in phosphate-buffered saline and then stained with Annexin V-FITC (Biovision, Mountainview, CA) and propidium iodide (PI, Sigma-Aldrich, Saint Louis, MO) according to the manufacturer's instructions. After staining with annexin V-FITC and PI, samples were analyzed by fluorescence-activated cell scanner (FACScan) flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Construction of PA28γ plasmid and cell transfection
A cDNA encoding human PA28γ was generated by polymerase chain reaction (PCR) from human brain cDNA library (Invitrogen, Carlsbad, CA) and subcloned into the eukaryotic expression plasmid pcDNA3 (pcDNA3-PA28γ). ALVA411 cells were transfected with pcDNA3-PA28γ or an empty vector (pcDNA3) using Lipofectamine 2000 according to the protocol of the manufacturer.
Western blot analysis
Cells were lysed in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton-X100 and protease inhibitor cocktail (Sigma-Aldrich, Saint Louis, MO). Cell extract protein amounts were quantified using the BCA protein assay kit. Equivalent amounts of protein (25 µg) were separated using 12% SDS-PAGE and transferred to PVDF membrane (Millipore Corporation, Billerica, MA). Western immunoblotting was performed using primary antibodies against 19S S1-8 (Thermo Scientific Pierce Antibodies), 20S C2 (Thermo Scientific Pierce Antibodies), 20S LMP7 (Thermo Scientific Pierce Antibodies), PA28α,β,γ (Alexis) or tubulin (Sigma-Aldrich), horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (Amersham Biosciences, UK) and ECL solutions (Amersham Biosciences, UK).
Posttranslational modification (PTM) prediction methods
To predict potential of O-GlcNAc/phosphorylation modification and Yin Yang sites in human PA28γ, artificial neural networks YinOYang 1.2 (http://www.cbs.dtu.dk/services/YinOYang) and Netphos 2.0 (http://www.cbs.dtu.dk/services/Netphos) were used to predict potential Yin Yang sites and phosphorylation sites, respectively, as previously reported (Kaleem et al., 2009).
Small interfering RNA (siRNA)
The sequence of siRNA against OGT (siOGT) was GAAGAAAGUUCGUGGCAAA. The scramble nonsense siRNA (scramble; CCGUAUCGUAAGCAGUACU) that has no homology to any known genes was used as control. Transfection of siRNA oligonucleotide was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations.
Data analysis
Statistical difference were evaluated using ANOVA with Dunnett's post hoc test and considered significant at P < 0.05.
Acknowledgements
This work was partly supported by National Natural Science Foundation of China (30870522, 31070697) and Foundation of Liaoning Educational Committee (L2010561) to H-Q Wang.
Abbreviations
- AMC
7-amino-4-methylcoumarin
- FACScan
fluorescence-activated cell scanner
- GFAT
glutamine:fluctose-6-phosphate amidotransferase
- HBP
hexosamine biosynthetic pathway
- O-GlcNAc
O-linked β-N-acetyglucosamine
- OGT
O-GlcNAc transferase
- PTM
posttranslational modification
References
- 1.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. 2005;43:187–201. doi: 10.1016/j.fct.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Ball HA, Wick AN, Sanders C. Influence of glucose antimetabolites on the Walker tumor. Cancer Res. 1957;17:235–239. [PubMed] [Google Scholar]
- 4.Biggee BA, McAlindon T. Glucosamine for osteoarthritis: part II, biologic and metabolic controversies. Med Health R I. 2004;87:180–181. [PubMed] [Google Scholar]
- 5.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 6.Fjelde A, Sorkin E, Rhodes JM. The effect of glucosamine on human epidermoid carcinoma cells in tissue culture. Exp Cell Res. 1956;10:88–98. doi: 10.1016/0014-4827(56)90075-1. [DOI] [PubMed] [Google Scholar]
- 7.Hagemann C, Patel R, Blank JL. MEKK3 interacts with the PA28 gamma regulatory subunit of the proteasome. Biochem J. 2003;373:71–79. doi: 10.1042/BJ20021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 10.Kaleem A, Ahmad I, Hoessli DC, Walker-Nasir E, Saleem M, Shakoori AR, Nasir Ud D. Epidermal growth factor receptors: function modulation by phosphorylation and glycosylation interplay. Mol Biol Rep. 2009;36:631–639. doi: 10.1007/s11033-008-9223-6. [DOI] [PubMed] [Google Scholar]
- 11.Kizer DE, Mc CT. The synthesis of hexosamine in tumor homogenates. Cancer Res. 1959;19:307–310. [PubMed] [Google Scholar]
- 12.Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. doi: 10.1038/sj.onc.1203043. [DOI] [PubMed] [Google Scholar]
- 13.Lingbeck JM, Trausch-Azar JS, Ciechanover A, Schwartz AL. In vivo interactions of MyoD, Id1, and E2A proteins determined by acceptor photobleaching fluorescence resonance energy transfer. FASEB J. 2008;22:1694–1701. doi: 10.1096/fj.07-095000. [DOI] [PubMed] [Google Scholar]
- 14.Liu K, Paterson AJ, Zhang F, McAndrew J, Fukuchi K, Wyss JM, Peng L, Hu Y, Kudlow JE. Accumulation of protein O-GlcNAc modification inhibits proteasomes in the brain and coincides with neuronal apoptosis in brain areas with high O-GlcNAc metabolism. J Neurochem. 2004;89:1044–1055. doi: 10.1111/j.1471-4159.2004.02389.x. [DOI] [PubMed] [Google Scholar]
- 15.Luhrs W. A contribution to the question glucosamine on the growth retardation of tumours. Acta Unio Int Contra Cancrum. 1957;13:480–481. [PubMed] [Google Scholar]
- 16.Marlow AA, Bartlett GR. Glucosamine and leukemia. Proc Soc Exp Biol Med. 1953;84:41–43. doi: 10.3181/00379727-84-20534. [DOI] [PubMed] [Google Scholar]
- 17.Marshall S, Nadeau O, Yamasaki K. Dynamic actions of glucose and glucosamine on hexosamine biosynthesis in isolated adipocytes: differential effects on glucosamine 6-phosphate, UDP-N-acetylglucosamine, and ATP levels. J Biol Chem. 2004;279:35313–35319. doi: 10.1074/jbc.M404133200. [DOI] [PubMed] [Google Scholar]
- 18.Masson E, Wiernsperger N, Lagarde M, El Bawab S. Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides. Biochem J. 2005;388:537–544. doi: 10.1042/BJ20041506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complications. 2002;16:72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 20.Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y, Tanaka K, Chiba T. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274:38211–38215. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 21.Qian J, Steigerwald K, Combs KA, Barton MC, Groden J. Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis. Oncogene. 2007;26:4872–4876. doi: 10.1038/sj.onc.1210265. [DOI] [PubMed] [Google Scholar]
- 22.Quastel JH, Cantero A. Inhibition of tumour growth by D-glucosamine. Nature. 1953;171:252–254. doi: 10.1038/171252a0. [DOI] [PubMed] [Google Scholar]
- 23.Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 25.Roessler M, Rollinger W, Mantovani-Endl L, Hagmann ML, Palme S, Berndt P, Engel AM, Pfeffer M, Karl J, Bodenmuller H, Ruschoff J, Henkel T, Rohr G, Rossol S, Rosch W, Langen H, Zolg W, Tacke M. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics. 2006;5:2092–2101. doi: 10.1074/mcp.M600118-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Sorkin E, Fjelde A. The effect of D-glucosamine and related products on human cancer cells in tissue culture. G Ital Chemioter. 1956;3:355–361. [PubMed] [Google Scholar]
- 27.Su K, Yang X, Roos MD, Paterson AJ, Kudlow JE. Human Sug1/p45 is involved in the proteasome-dependent degradation of Sp1. Biochem J. 2000;348(Pt 2):281–289. [PMC free article] [PubMed] [Google Scholar]
- 28.Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radic Biol Med. 2008;45:50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Thomas SM, Coppelli FM, Wells A, Gooding WE, Song J, Kassis J, Drenning SD, Grandis JR. Epidermal growth factor receptor-stimulated activation of phospholipase Cgamma-1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 2003;63:5629–5635. [PubMed] [Google Scholar]
- 30.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem. 2007;282:22460–22471. doi: 10.1074/jbc.M702439200. [DOI] [PubMed] [Google Scholar]