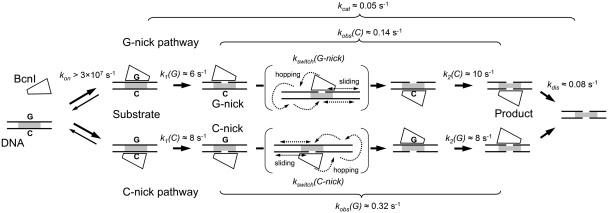
Figure 7.
Mechanism of double-stranded DNA cleavage by BcnI. BcnI rapidly associates with substrate DNA (rate constant kon) placing the catalytic center in the vicinity of either the C- (5′-CCCGG-3′) or the G- (5′-CCGGG-3′) strand. Rapid hydrolysis of the first DNA strand [rate constants k1(G) and k1(C)] converts the substrate into a mixture of G- and C-nick intermediates. The second strand is cleaved at a much lower rate [rate constants kobs(C) and kobs(G)]. The latter process consists of two phases: (i) a relatively slow switch of BcnI on DNA [rate constants kswitch(G-nick) and kswitch(C-nick)] involving a diffusional walk on DNA; and (ii) a rapid hydrolysis reaction [k2(C) and k2(G)]. BcnI preferentially binds intact DNA in the orientation that places the catalytic center close to the G-strand, therefore the major fraction of DNA (70–80%) is cleaved via the G-nick intermediate. The reaction cycle is completed by the product release (rate constant kdis). The rate constants for cleavage of the first and the second DNA strands together with kdis define the turnover number of BcnI kcat. Occasional dissociation of BcnI from the nicked intermediate (rate constant ∼0.02 s−1) is not depicted.