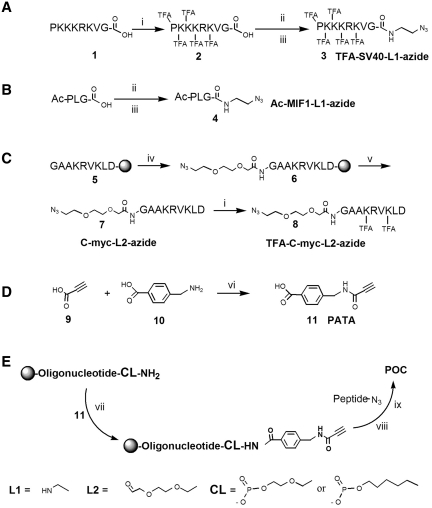
Scheme 1.
(A–C) Synthesis of azido-functionalized peptides (D) Synthesis of active alkyne linker, PATA. (E) Scheme for POC synthesis on solid support using commercial oligonucleotides with aminolinkers. Reagents and conditions: (i) ethyl trifluoroacetate, methanol, 3 days, RT; (ii) preactivation with HBTU and NMM in DMF 0.5 h, RT; (iii) azidoethylamine, 2 h, RT; (iv) 2-(2-azidoethoxy)ethoxyacetic acid (preactivated with HBTU and NMM in DMF, 0.5 h, RT), DMF, 2 h, RT; (v) 90% TFA, 4% TIS, 4% H2O, 2% 3,5-dioxo-1,8-octanedithiol, 4 h, RT; (vi) preactivation of 9 with DCC, in acetonitrile, 0°C, then addition of 10; (vii) preactivation of 11 with HBTU, and NMM, DMF, 0.5 h, RT, then addition to solid supported oligonucleotide 2 h; (viii) 1.2 eq. CuSO4, 3 eq. sodium ascorbate, 2 eq. of peptide-azide; overnight; (ix) NH3, (aq) sat. 55°C overnight (HBTU, O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate; azide, N3; Ac, acetyl; TIS, triisopropyl silane; TFA, trifluoroacetic acid).