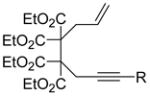
Table 2.
Scope of Enyne Cycloisomerizationa
| entry | enyne | product | yield (%)b,c |
|---|---|---|---|
| 1 |
|
||
| 1 R = Me | 2 | 90 | |
| 3 R = Et | 4 | 92 | |
| 5 R = nPr | 6 | 97 | |
| 7 R = iPr | 8 | 99 | |
| 2 |
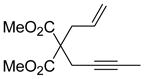 9 |
10 | 82 |
Reaction Conditions: 5 mol% Ni(COD)2, 10 mol% IDTB, 0.1M 1, 60 °C, 1h.
Isolated yield, average of two runs.
>95:5 E:Z based on 1H NMR analysis of crude reaction mixture.
