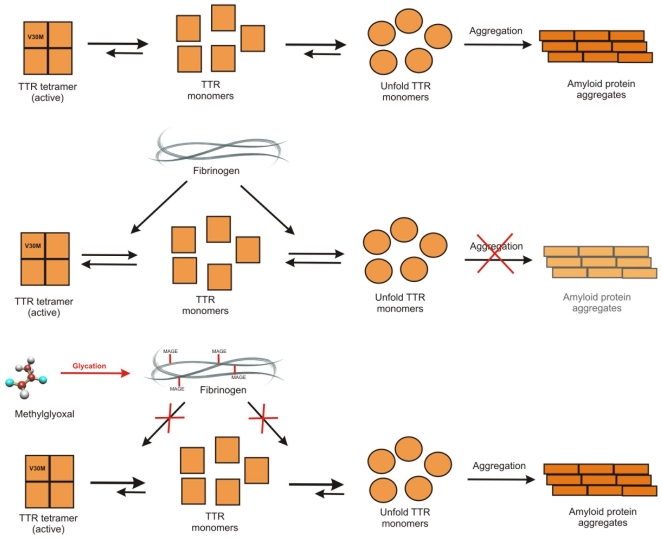
Figure 6. Molecular model of the effects of fibrinogen glycation in vivo in FAP.
In vitro studies established that amyloidogenic TTR variants (as V30M) have a higher tendency to follow an aggregation pathway high the formation of amyloid deposits. Considering our findings that fibrinogen interacts with TTR in the plasma, we proposed that fibrinogen chaperone activity reduces TTR propensity towards fibril formation. However, the specific glycation of fibrinogen compromises its vital chaperone activity and in these conditions TTR becomes more prone to aggregation.