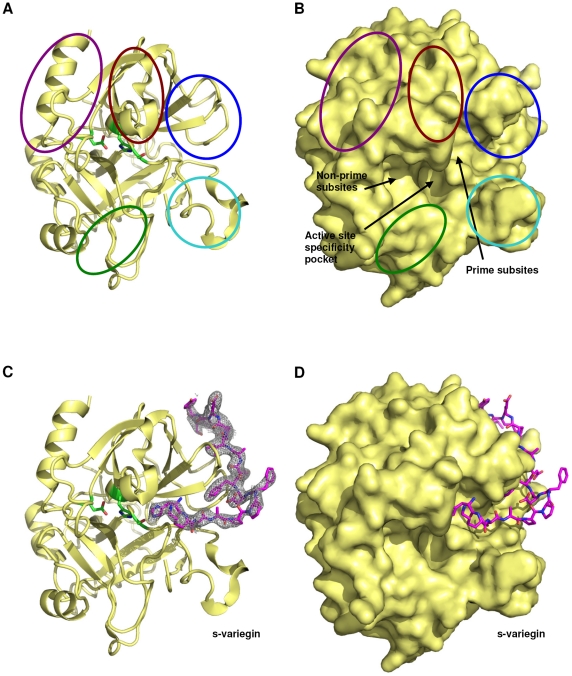
Figure 1. Structure of thrombin:s-variegin complex.
(A) Thrombin (yellow) shown in the classical orientation in ribbon (without s-variegin). Side chains of catalytic triad, TAsp102, THis57 and TSer195 are shown in sticks (green). The 60-loop, autolysis loop and Na+-binding loop are circled in brown, cyan and green, respectively. Parts of thrombin forming the anion-binding exosite-I and exosite-II are circled in blue and purple, respectively. (B) Surface representation of thrombin (yellow) in the same orientation as (a). Locations of active site specificity pocket, non-prime and prime subsites are indicated by arrows. (C) The structure of thrombin (yellow) in the same orientation as above shown in complex with s-variegin (pink) together with its electron density map (2Fo-Fc) shown contoured at 0.9σ. (D) Surface representation of thrombin in complex with s-variegin (pink).