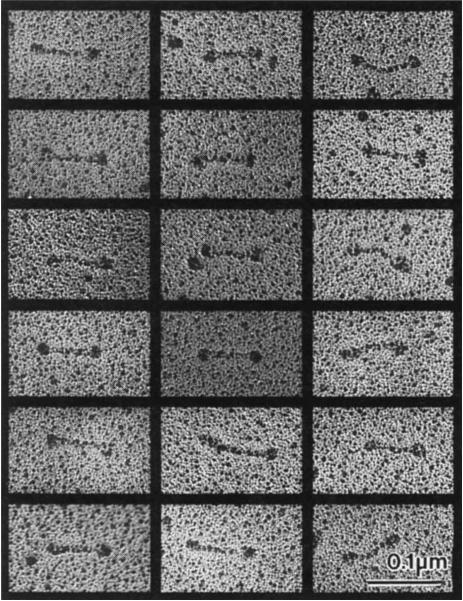
FIG. 2.
Electron micrographs of rotary-shadowed KPR130 molecules. These images, together with previous studies13, are consistent with the hypothesis that a KRP130 holoenzyme comprises four 130K subunits assembled into a structure 96 nm long, with a total Mr of roughly 500K, Stokes radius of 16.2 nm, and S-value of 7.6 S. For comparison, kinesin comprises two heavy chains of 110K–130K and two light chains of 55K–80K assembled into a structure 75–80 nm long with a total Mr of 350K–400K, Stokes radius of 9nm, and S-value of 9.0–9.6S. The dimensions of both rotary-shadowed KRP130 and kinesin appear slightly larger than their true dimensions owing to the coating of platinum, which in our experiments is routinely estimated to be approximately 2.5 nm thick.
METHODS. KRP130 alone or KRP130–MT complexes were mixed with an equal volume of 80% glycerol containing 0.6–1.0 M ammonium acetate and sprayed onto freshly cleaved mica plates. The plates were then vacuum dried, rotary shadowed with platinum at a 6° angle using a Balzers BAF 400T freeze-fracture device, and processed as described previously28. The replicas were visualized on a Philips 410LS electron microscope at 80 kV. No difference in the structure of KRP130 was observed with or without the MT binding step.