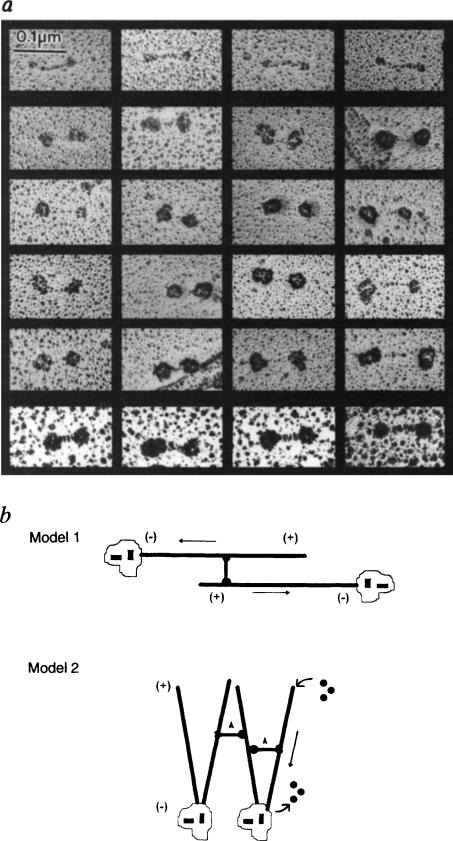
FIG. 4.
Antibody decoration of bipolar KRP130 tetramers and models of KRP130 function, a, Rotary-shadowed KRP130 decorated with anti-Eg5 motor-domain antibody (Fig. 1c). About one-quarter of the molecules on the grid were undecorated (top row). Decorated molecules show the characteristic ‘dumbbell’ shape but the heads are enlarged. Because of the large size of the heads and low angle of shadowing, the contrast of the ‘rod’ of decorated molecules (middle four rows) is hard to visualize, but was clearer when rotary shadowing was prolonged to produce larger platinum grain (bottom row). b, Models for KRP130 function. In model 1, KRP130 crosslinks two antiparallel MTs emanating from opposite spindle poles with MT plus ends distal to the poles. The motor domains at opposite ends of KRP130 molecules move towards the plus ends of the crosslinked MTs, causing the MTs themselves to slide apart, minus ends leading (arrows), resulting in spindle-pole separation during the formation, maintenance or elongation of a bipolar mitotic spindle. In model 2, KRP130 could crosslink parallel MTs emanating from one or both poles and drive movements necessary for spindle formation and maintenance. For example, by moving towards the plus ends of crosslinked MTs (arrowheads) it may ‘zip’ together MTs into kinetochore fibres or, as proposed for Xenopus Eg5 (ref. 5), it could exert pushing forces (straight arrow) that drive MT flux towards the poles and take part in organizing spindle poles. We favour model one, which is more consistent with the mechanism of action of the bipolar actin-based motor, myosin II.
METHODS. Purified KRP130 from the sucrose gradients was mixed with MTs and anti-Eg5 motor-domain antibody (Fig. 1) in amounts approximately equimolar to the amount of KRP130. The MT–KRP130–antibody complexes were pelleted through a glycerol cushion and processed for rotary shadowing as described (Fig. 2 legend).