Figure 6.
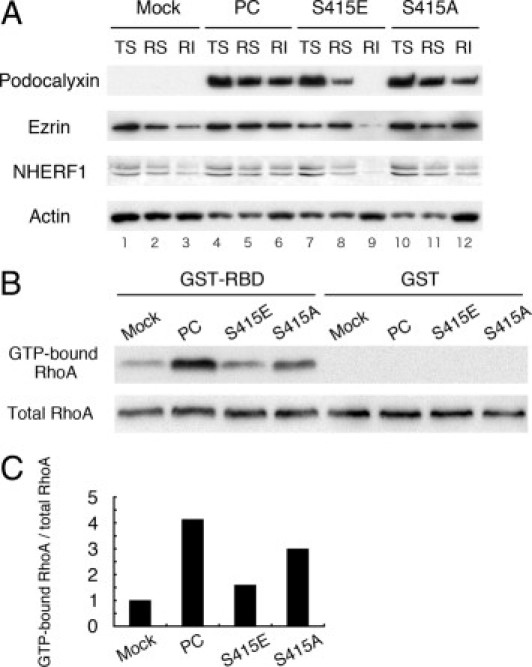
Dissociation of PC, ezrin, and NHERF1 from the actin cytoskeleton in MDCK-S415E cells expressing a phosphomimetic PC mutant. A: In wild-type MDCK-PC cells, PC is distributed in the Triton X-100 soluble (TS), RIPA soluble (RS), and RIPA insoluble (RI) fractions (lanes 4, 5, and 6, respectively) as shown previously.9 By contrast, in the phosphomimetic MDCK-S415E cells, PC is mainly detected in the TS fraction (lane 7) and is greatly reduced in the RS fraction (lane 9). The amount of PC found in the RI fraction is also reduced in the phospho-deficient MDCK-S415A (lanes 10–12). Ezrin and NHERF1 are also distributed in all three fractions in MDCK-PC cells (lanes 4–6), but the amount found in the RI fraction is decreased in the phosphomimetic MDCK-S415E cells (lane 9) and MDCK-mock (lane 3) compared with the MDCK-PC (lane 6) and MDCK-S415A cells (lane 12). MDCK cells were sequentially extracted in Triton X-100 and RIPA buffer as described in Materials and Methods, and equal volumes of each fraction were immunoblotted for PC, ezrin. and actin. B: Amount of GTP-bound active RhoA (upper panel) is higher in MDCK-PC and MDCK-S415A than in MDCK-mock and MDCK-S415E cells. The amount of total RhoA (lower panel) is similar in all cell lines. GST-RhoA binding domain (GST-RBD) was incubated with 700 μL cell lysate, and total RhoA and GTP-bound RhoA were detected with anti-RhoA IgG (1:200). As a negative control, lysates were also incubated with GST alone (GST). C: Densitometric analysis reveals that RhoA activity in MDCK-PC and phospho-deficient MDCK-S415A cells is approximately fourfold and threefold higher, respectively, than in MDCK-mock cells whereas RhoA activity in the phosphomimetic MDCK-S415E cells is greatly reduced. The relative RhoA activity was calculated from the ratios of GTP-bound RhoA/total RhoA and normalized to the ratio obtained for MDCK-mock cells.
