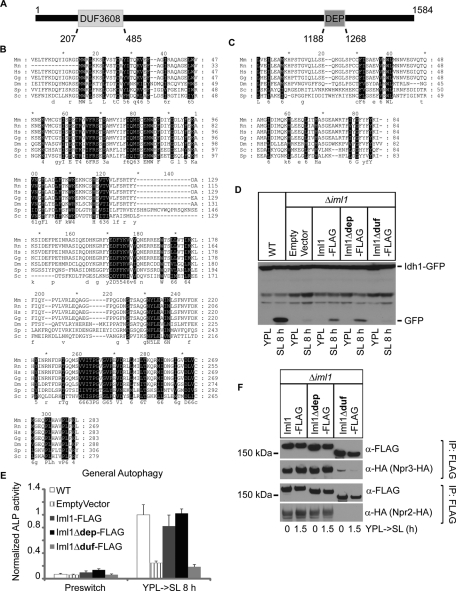
FIGURE 8:
Iml1p contains an evolutionarily conserved domain that is important for NNS-induced autophagy and formation of the Iml1p complex. (A) A schematic representation of the domain structure of Iml1p. (B and C) Multiple sequence alignment for the DUF domain (B) and the DEP domain (C) between Iml1p and its orthologues. Highly conserved residues are in black. Mm, Mus musculus; Rn, Rattus norvegicus; Hs, Homo sapiens; Gg, Gallus gallus; Dm, Drosophila melanogaster; Sp, Schizosaccharomyces pombe; Sc, Saccharomyces cerevisiae. (D and E) The DUF domain of Iml1p is required for NNS-induced autophagy as assessed by Western blot and ALP activity assay. The coding sequence for full-length IML1-FLAG, iml1Δdep-FLAG, or iml1Δduf-FLAG was cloned into a centromeric plasmid under the control of the CYC1 promoter. Empty vector or plasmids expressing Iml1-FLAG, Iml1Δdep-FLAG, or Iml1Δduf-FLAG were transformed into Δiml1 cells to determine whether they could rescue NNS-induced autophagy. WT cells are shown for comparison. In (E), data represent averages of three to five samples, with error bars for standard deviations. (F) The DUF (RANS) domain of Iml1p is required for its interaction with Npr2p and Npr3p. Empty vector or plasmids expressing Iml1-FLAG, Iml1Δdep-FLAG, or Iml1Δduf-FLAG were transformed into Δiml1 cells and then used to pull down Npr2p or Npr3p.