Figure 1.
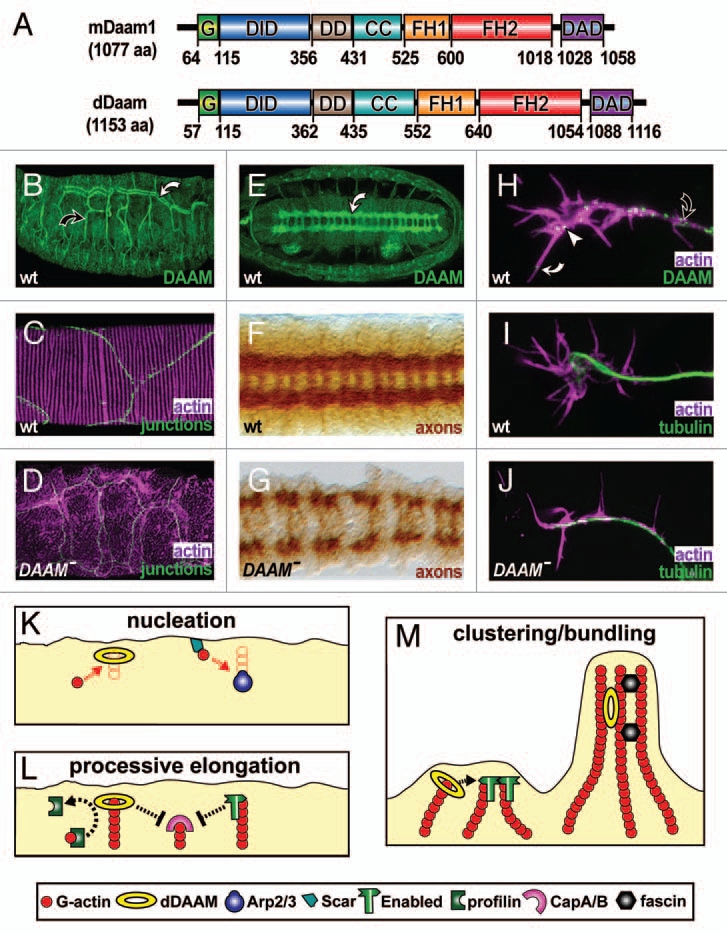
Properties and functions of Drosophila DAAM. (A) Mouse and Drosophila DAAM are of similar length and display the same functional domains: GTPase binding (G), diaphanous inhibitory (DID), N-terminal dimerisation (DD), coiled-coil (CC), formin homology 1 and 2 (FH1, FH2), diaphanous autoregulatory domain (DAD); residues demarcating the functional domains are shown below. (B) In embryos, dDAAM is strongly expressed in main (white curved arrow) and side branches (black curved arrow) of tracheal trees. (C) At high magnification, tracheae show parallel lines of F-actin enrichment (magenta) that run across cellular junctions (green, stained for De-Cadherin) in the main airways. (D) In tracheae of dDAAM loss-of-function mutant embryos, ordered F-actin patterns are abolished. (E) dDAAM is strongly expressed in the ladder-shaped neuropile within the embryonic CNS (white curved arrow). (F) In wild-type embryos, the axonal marker BP102 labels the ladder-like arrangement of the neuropile. (G) In dDAAM loss-of-function mutant embryos, the neuropile is severely disrupted indicating strong axonal growth defects. (H) In cultured primary embryonic neurons, dDAAM displays a punctate pattern along the axon (black curved arrow), but also at the growth cone (white arrow head) and in filopodia (white curved arrow). (I) In wild-type neurons, growth cones frequently display a hand-shaped broad appearance. (J) In dDAAM loss-of-function mutant neurons, growth cones tend to be narrow and display significantly reduced numbers of filopodia. (K–M) Potential functions of dDAAM during filopodia formation in Drosophila neurons. (K) dDAAM acts as an actin nucleator in parallel to Scar complex/Arp2/3 complex activity. (L) Like enabled, dDAAM promotes processive elongation of actin filaments, expected to collaborate with profilin and potentially antagonising the capping activities of the CapA/B complex. (M) The ability to bind and bundle actin filaments in vitro25 suggests potential roles for dDAAM in clustering of actin filament barbed ends (together with enabled; left) or the stabilisation of F-actin bundles (together with other bundlers, such as fascin; right).
