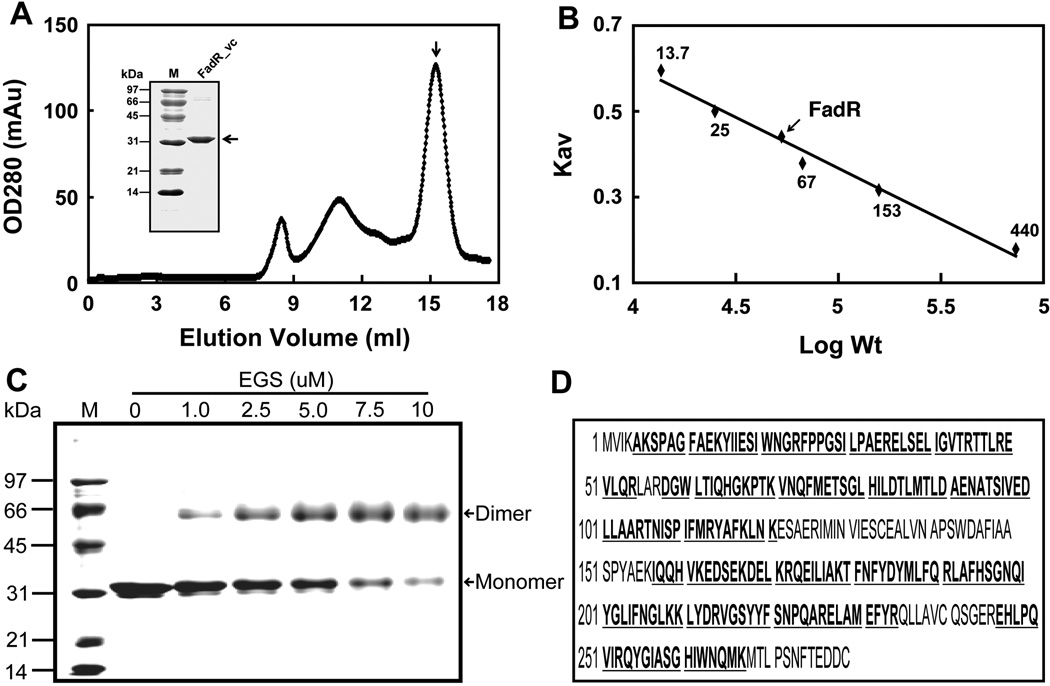
Fig. 2. Biochemical and structural characterization of V. cholerae FadR.
A. Gel exclusion chromatographic profile of recombinant V. cholerae FadR run on a Superdex 200HR 10/30 column (GE Healthcare). The expected peak of purified V. cholerae FadR was eluted at the position of 15.2 ml (indicated with an arrow). The inset gel is the SDS-PAGE analysis of the purified V. cholerae FadR. The apparent molecular weight of recombinant V. cholerae FadR is about 31 kDa. OD280, optical density at 280 nm; mAu, milli-absorbance units.
B. Determination of V. cholerae FadR solution structure according to elution patterns of a series of standard proteins (Pharmacia). The standard proteins were ribonuclease (~13.7 kDa), chymotrypsinogen (~25 kDa), bovine serum albumin, 67 kDa), aldolase (153 kDa), and ferritin (~440 kDa). The elution position of V. cholerae FadR is indicated with an arrow. KAV, partition coefficient; M, molecular weight.
C. Chemical cross-linking assay of V. cholerae FadR solution structure.
D. MS identification of V. cholerae FadR. The matched amino acid residues are given bold and underlined type.