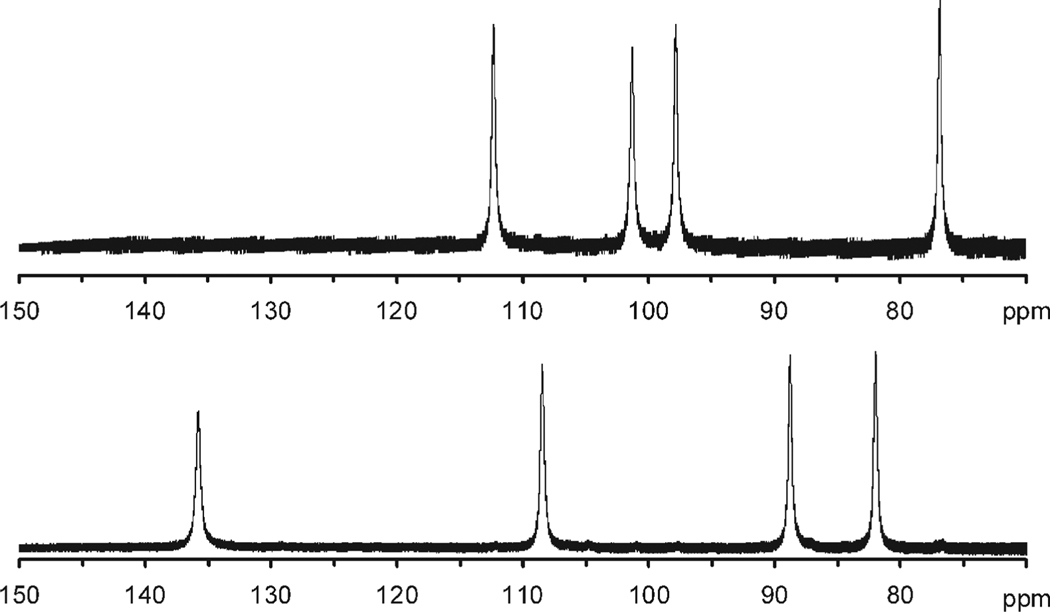
Figure 2.
Expansion of the hyper-shifted region of the 1H NMR spectra (300 MHz, D2O) of two isomeric chelates of YbNB-DOTMA (both TSAP coordination isomers) focusing on the axS protons of the macrocyclic ring. Previously these two isomers were ascribed to the S-SSSS- (top) and S-RSSS- (bottom) isomers.7,24 This assignment is now understood to be erroneous, in fact these spectra correspond to two regioisomers of the S-SSSS- isomer in which the nitrobenzyl substituent is located on the corner (top) and on the side (bottom) of the macrocyclic ring, see Figure 1.