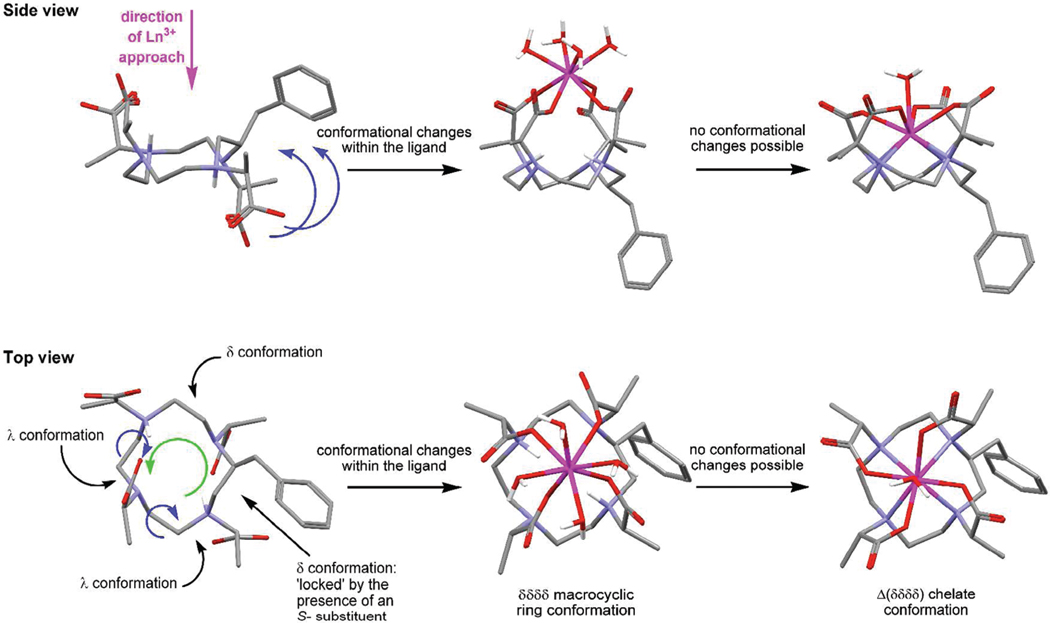
Figure 8.
Conformational changes that are hypothesized to occur during the formation of a chelate that positions the benzylic substituent on the side of the macrocycle, shown for the S-SSSS- isomer of NB-DOTMA. The ligand structure is derived from the crystallographic data for RRSS-TCE-DOTA and the benzylic substituent located in the position found to afford the lowest energy conformation, vide infra. To flip the two pendant arms in the right-hand side of the ligand above the plane of the macrocycle (as shown) it is necessary for each atom in the macrocycle to undergo a positional shift (green arrow) which enable the two ethylene bridges in a λ conformation to flip conformations while flipping the pendant arms on the right into a position above the ring (blue arrows). This positional shift also moves the benzylic substituent from the corner (in the ligand) to the side of the macrocycle (in the chelate).