Figure 7.
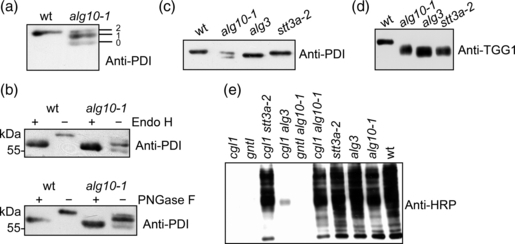
alg10-1 displays a severe underglycosylation defect.
(a) Immunoblot analysis of protein disulfide isomerase (PDI). Protein extracts were separated by SDS-PAGE and blots were analyzed with anti-PDI antibodies. The positions of the fully glycosylated PDI form (2), the form with one N-glycan (1) and the non-glycosylated PDI form (0) are given.
(b) Protein extracts from wild-type (wt) and alg10-1 were subjected to Endo H and PNGase F treatment to confirm the underglycosylation status of PDI in alg10-1.
(c) Protein gel blot analysis of PDI from different underglycosylation mutants (alg10-1, alg3, stt3a-2).
(d) Total proteins from wild-type, alg10-1, alg3 and stt3a-2 were separated by SDS-PAGE and blots were analyzed with anti-TGG1 antibodies. The shift in mobility of PDI and TGG1 in alg3 is caused by the aberrant truncated N-glycan structures present in this mutant (Henquet et al., 2008; Kajiura et al., 2010).
(e) Total proteins from the indicated single (cgl1, gntI, stt3a-2, alg3, alg10-1) and double mutants (cgl1 stt3a-2, cgl1 alg3, gntI alg10-1 and cgl1 alg10-1) were analyzed with anti-horseradish peroxidase (anti-HRP) antibodies.
