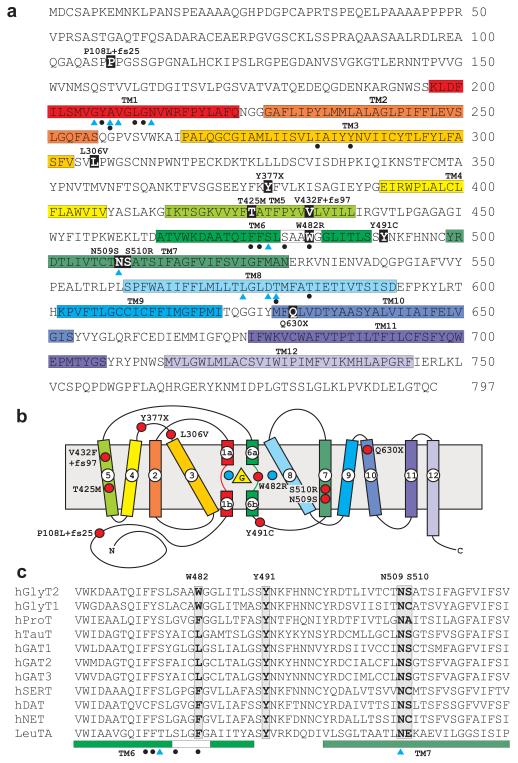
Fig. 1. Amino acid sequence of human GlyT2 indicating mutations identified in hyperekplexia.
a, Amino acid sequence of human GlyT2 indicating the revised positions of putative transmembrane (TM) domains (coloured boxes). Blue triangles show residues in hGlyT2 that are likely to co-ordinate Na+ ions based on sequence alignments with the bacterial leucine transporter LeuT24. This involves residues in TM1 (G206, A208, V209, N213), TM6 (S477), TM7 (N509) and TM8 (L574, D577, T578). However, it is noteworthy that GlyT2 binds three Na+ ions, while LeuT binds two, suggesting that other residues involved in Na+ co-ordination remain to be identified. Filled black circles indicate residues predicted to be involved in glycine binding, including residues in TM1 (Y207, A208, L211, G212), TM3 (I283, Y287), TM6 (F475, F476, S479, W482) and TM8 (T578, T582). b, Schematic diagram showing the suggested topology of GlyT2 and relative positions of GlyT2 mutations (red circles), adapted from Yamashita et al24. The positions of glycine and two of the three sodium ions are depicted by a yellow triangle and blue circles, respectively. c, alignment of the TM6-TM7 region of GlyT2 with other members of the Na+/Cl−-dependent neurotransmitter transporters reveals that residue W482 is only found at the equivalent position in GlyT1 and GlyT2, while Y491 and N509 are highly conserved throughout this superfamily. Residue S510 is conserved in taurine, GABA and dopamine transporters.