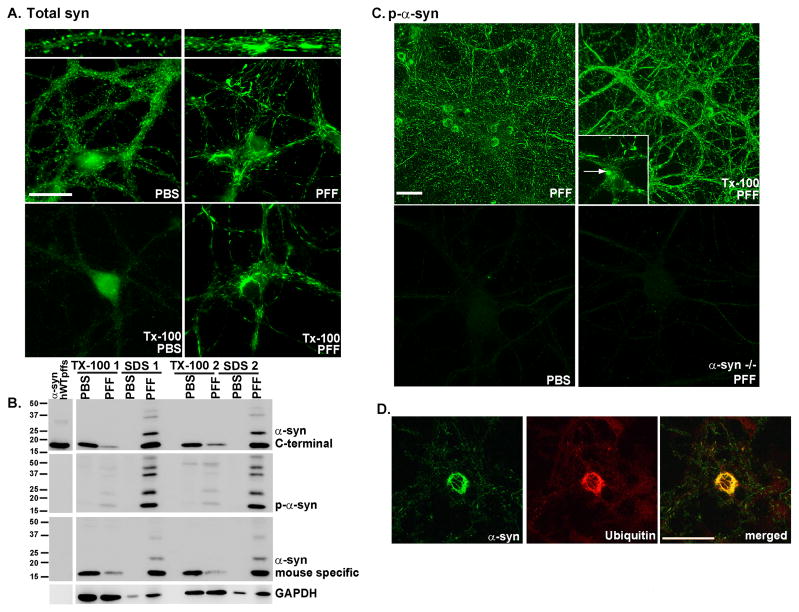
Figure 1. α-syn-hWT pffs recruit endogenous α-syn to form pathologic, insoluble aggregates.
A. Two weeks following pff addition, neurons were fixed with paraformaldehyde alone or paraformaldehyde with 1% Tx-100 to extract soluble proteins. In PBS treated neurons, α-syn localized to the presynaptic terminal and was Tx-100 soluble. Addition of α-syn pffs formed Tx-100 insoluble aggregates which recruited α-syn away from synapses. Scale bar = 20 μm. B. Neurons were treated with α-syn-hWT pffs, and 2 weeks later, were sequentially extracted with 1% Tx-100 followed by 2% SDS. Shown are immunoblots from 2 independent sets of samples. Antibodies that either recognize the C-terminus of α-syn (top) or are specific for mouse α-syn (bottom) showed that in PBS treated neurons, α-syn was soluble in Tx-100. α-Syn-hWT pff treatment reduced soluble α-syn and increased Tx-100-insoluble α-syn. The first lane shows α-syn-hWT pffs alone to demonstrate that the C-terminal antibody recognizes both human and mouse α-syn, and the mouse specific antibody recognizes only mouse pffs. Furthermore, the α-syn-hWT pffs themselves are not phosphorylated. C. Addition of α-syn pffs increased pathologic, p-α-syn. Fixation with paraformaldehyde/Tx-100 demonstrated that the phosphorylated aggregates were insoluble. Scale bar = 50 μm. Insert: Within the somata, aggregates appear as LB-like skein-like filaments and dense inclusions (arrow). PBS- treated neurons and neurons from α-syn −/− mice did not show p-α-syn. D. The phosphorylated aggregates are ubiquitin positive (N=2). Scale bar = 50 μm. See also Supplementary Figure 1.