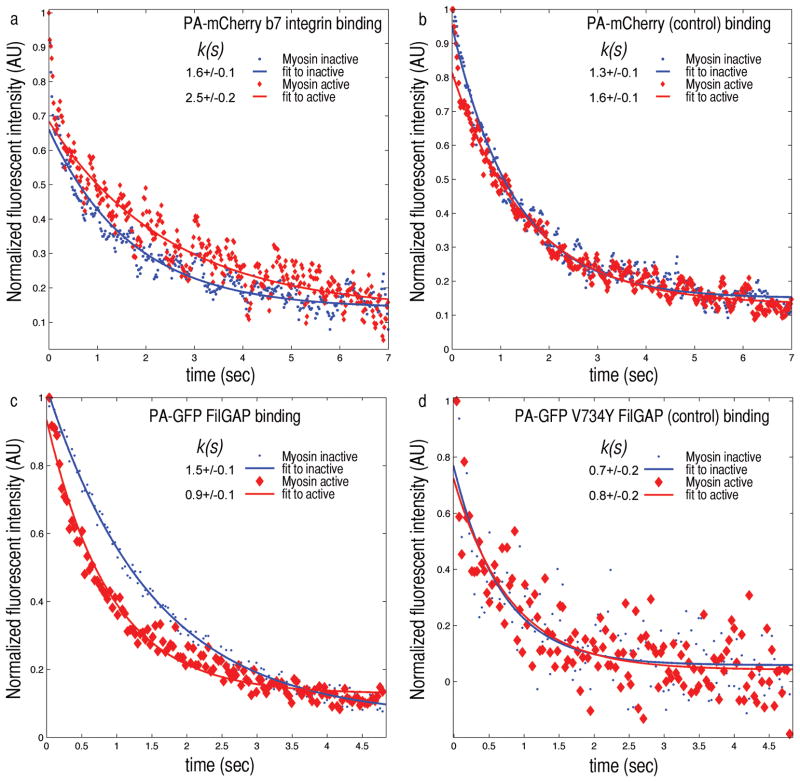
Figure 3. Myosin II forces applied to F-actin-FLNa networks changes FLNa’s binding affinity to β7 integrin and FilGAP.
a) When depleted of ATP, myosin is in a rigor state. The FLNa within the network is not stressed and PA-GFP β7 integrin fluorescence decays with a characteristic time constant k(s) of 1.6 seconds (blue). After caged ATP is released myosin reactivates, straining FLNa crosslinks. The decay time constant increases to 2.5 seconds as the integrin dissociates more slowly from FLNa under stress. b) PA-mCherry alone as a control shows no significant difference in the unstrained or strained state. c) Fluorescence intensity in time of PA-GFP FilGAP after photoactivation. In the ATP depleted state FilGAP’s fluorescence decay time k is 1.5 s, and after releasing the caged ATP (red) k decreases to 0.9 s. PA-GFP V734Y FilGAP, a non-FLNa binding mutant as a control, shows no significant difference in the decay times of unstrained (0.7 s) or strained state (0.8 s) (n=20).