Figure 6.
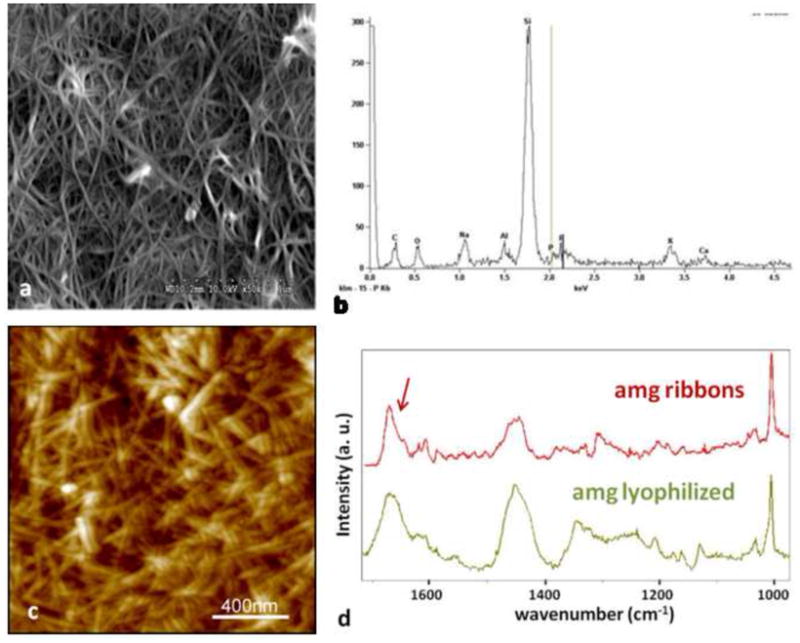
Large amounts of amelogenin ribbons were observed by SEM (a) and elemental analysis by EDX confirmed the presence of small amounts of Calcium and Phosphate ions in these structures which were deposited onto a silica based microscope slide (b). The more randomly distributed ribbons were also observed by AFM but only in areas were large amounts of the protein could be immobilized to the glass surface (c). Analysis of the fibrillar structures using micro-Raman spectroscopy showed large similarities between the lyophilized amelogenin powder as received after purification and the self-assembled structures deposited onto a glass-slide. Overall the spectrum obtained from the amelogenin ribbons appeared more pronounced with respect to the amide vibrational modes above 1400 cm−1. In particular the sharpening of a band at 1670 cm−1 and the occurrence of a band around 1610 cm−1 are indicative of the formation of β-sheet structures.
