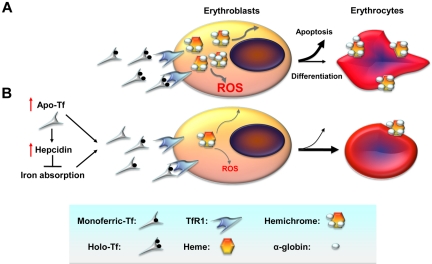
Figure 1.
Cellular mechanisms by which decreased iron uptake into erythroid precursors may promote survival and differentiation. (A) In β-thalassemia, a relative excess of α-globin synthesis leads to formation of hemichromes (α-globin/heme aggregates). Hemichromes are the primary cause of cellular toxicity in β-thalassemia because they precipitate and lodge on erythrocyte membranes, altering their structure. Furthermore, excess heme leads to the formation of reactive oxygen species (ROSs), which induce oxidative stress and cellular damage. In turn, this leads to IE by increasing apoptosis of erythroid precursors and reducing the number of erythrocytes produced as well as their survival in circulation. (B) On the basis of our data, we observe that administration of apo-Tf and increased hepcidin expression lead to decreased serum iron concentration and formation of fewer holo-Tf molecules. This reduces iron delivery to erythroid precursors, reducing heme synthesis and formation of hemichromes. In contrast, decreased iron intake limits hemichrome and ROS formation, ameliorating IE by reducing apoptosis, and improving erythrocyte survival in circulation. TfR1 indicates transferrin receptor 1.