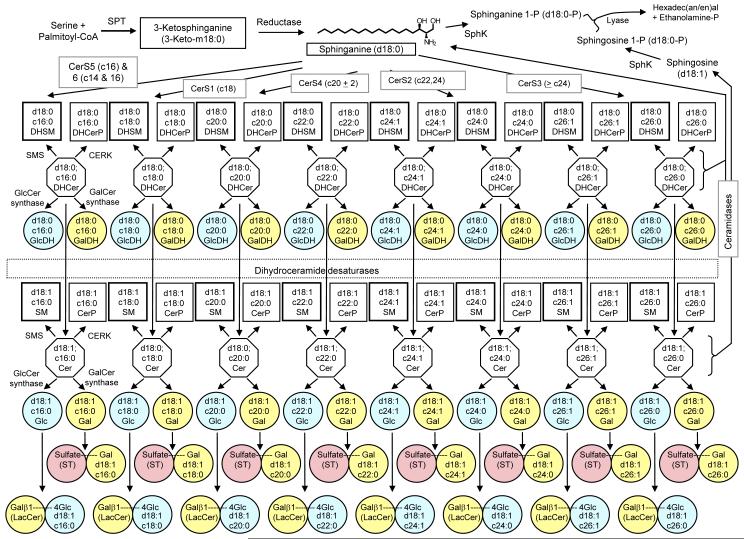
Figure 1.
Biosynthetic pathway of sphingolipids consisting of free long chain bases (top row), which are N-acylated by several CerS enzymes to form dihydroceramides (upper row of octagons). These DHCer may then processed by dihydroceramide desaturases to introduce a 4, 5 double bond to form ceramide (lower row of octagons). Collectively both the DHCer and Cer may be converted to more complex via addition of a polar headgroup at the 1′-OH position via sphingomyelin synthase to form sphingomyelin (thick lined squares), or ceramide kinase to form ceramide-1-phosphate (thin lined squares). Carbohydrates may also be linked to this position as well via glucosylceramide or galactosylceramide synthase to form glucosylceramides (light blue circles) and galactosylceramides (yellow circles), respectively. The former may have additional carbohydrates such as galactose complexed to it to form lactosylceramide (bottom row of light blue and yellow linked circles). The latter may also be sulfated to form the sulfatide species (pink circles). The far right and upper left corners show the degradative pathway of sphingolipids via ceramidases, sphingosine/sphinganine kinases, and sphingosine/sphinganine phosphate lyases, which constitutes the only known exit from the sphingolipid metabolic pathway.