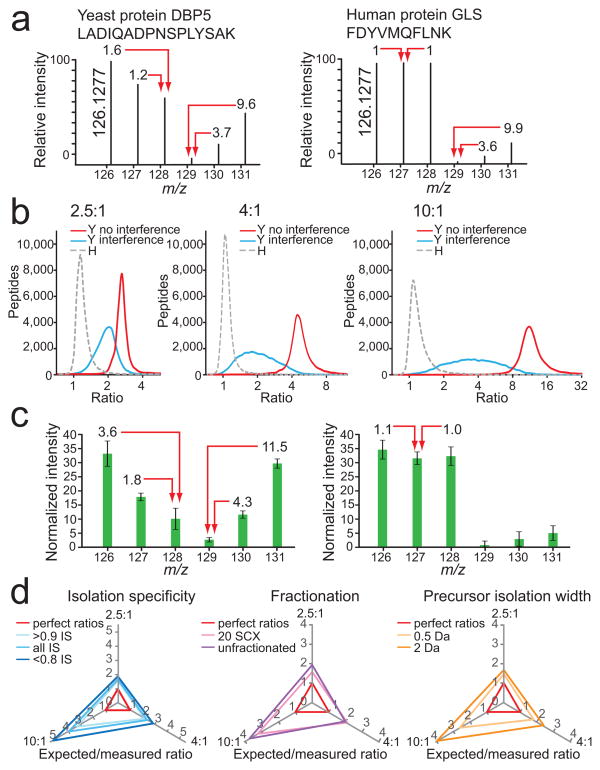
Figure 2.
Evaluation and attempted removal of the interference effect. (a) An example of strong distortion of reporter ion intensities for a yeast peptide ion by interference of human peptide ions (left) and the effect of interference by yeast peptides on the reporter ion intensities of a human peptide (right; signals in channels 129, 130, and 131). (b) Ratiodistributions (log2) of yeast (Y) peptides in channels with human (H) peptide interference (blue line), without human peptide interference (red line), and human peptides only (gray dashed line) for the predicted ratios 2.5:1 (left), 4:1 (middle), and 10:1 (right). LC-MS2 data were collected after separating the yeast and human whole cell lysate digest into 20 fractions using SCX. (c) Averaged normalized relative intensities for each TMT reporter ion channel for yeast peptides (left), and human peptides (right) from the dataset described in (b). Error bars represent one standard deviation for 20,272 and 81,180 yeast and human peptides, respectively. (d) There was minimal improvement of yeast peptide ratios in channels with human peptide interference (126, 127, and 128) with post-acquisition data filtering of isolation specificity (IS; left), fractionation (middle) and precursor isolation width (right). Expected ratios were divided by measured ratios, where perfect ratios would have a value of 1 for all three axes (red triangle). The greater the distance of each tested condition from the red perfect ratio triangle, the greater the influence of human peptide interference on yeast peptide ratio distortion. Increasing dynamic range results in greater ratio distortion.