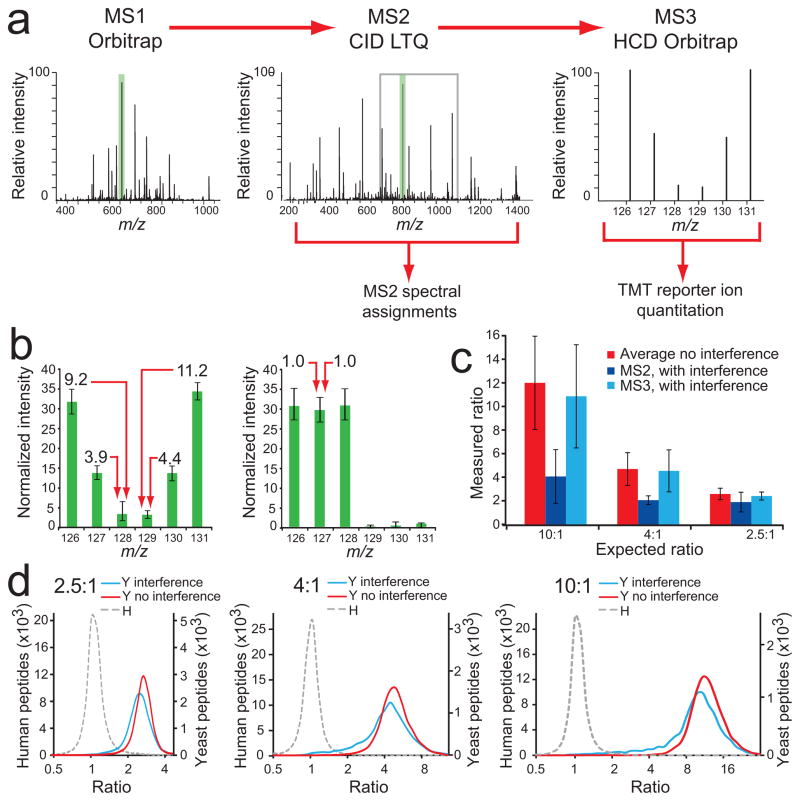
Figure 3.
An MS3-based method eliminates the interference effect. (a) Our MS3 method separates quantification from peptide identification. Fast LTQ-CID-MS2 experiments are used for peptide sequence assignments, followed by the selection of the most intense fragment ion from MS2 (green shading) for HCD-MS3, within a 110–160% m/z range relative to the precursor ion m/z value (gray box), where TMT reporter ion intensities are measured in the Orbitrap. (b) Normalized intensities for each TMT reporter ion channel for yeast peptides (left), and human peptides (right). Almost no interference effect was measured, except for forward false positives (1.5%). Error bars represent one standard deviation for 8,919 and 65,595 yeast and human peptides, respectively (c) Yeast peptide ratios from dataset combining 20 SCX fractions. Ratios were substantially compressed in channels containing interference when quantification was based on MS2 spectra. Using MS3 for quantification, measured yeast peptide ratios from channels also containing human peptides (126, 127, 128) were very close to ratios measured for channels that contained only yeast peptide ions (129, 130, 131). Error bars represent one standard deviation for 29,813, 22,640 and 7,173 peptides for Average, MS2 and MS3 analyses, respectively. (d) Ratio distribution (log2 scale) of yeast (Y) peptides in channels with human (H) peptide interference (blue line), without human peptide interference (red line), and for human peptides (gray dashed line) for 2.5:1 (left), 4:1 (middle), and 10:1 (right) combinations.