Figure 2.
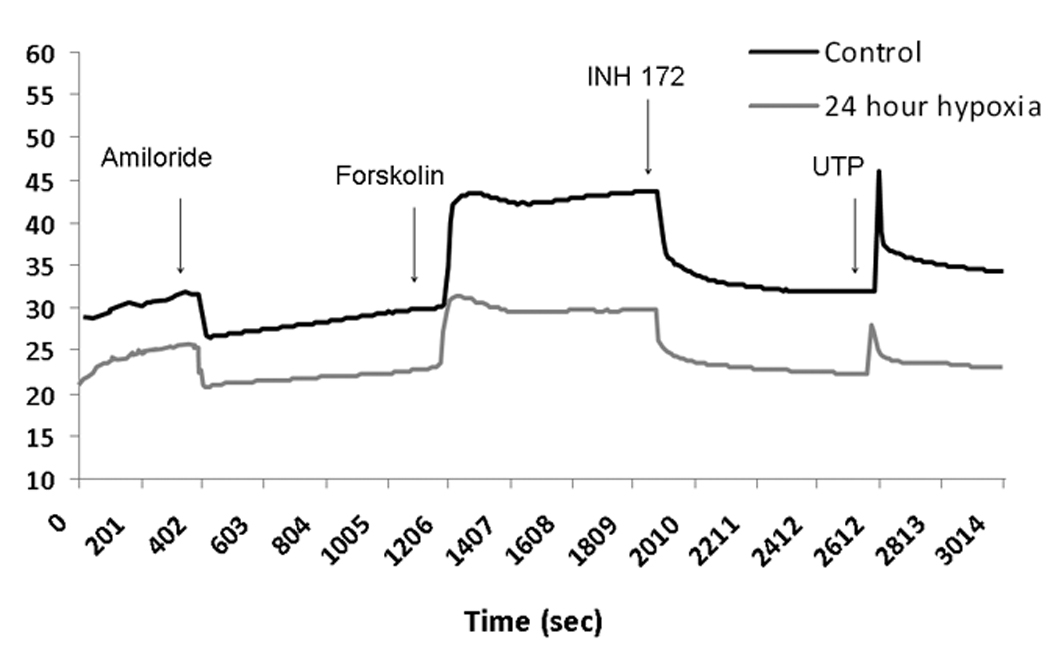
A: Representative ISC tracing of human sinonasal epithelial (HSNE) cultures. HSNE cells grown on transwell permeable supports and incubated in 1% O2 or physiologic O2 (control, 21%) were mounted in modified Ussing chambers under short-circuit conditions and sequentially exposed to amiloride (100µM); forskolin (20 µM); INH-172 (10 µM) and uridine triphosphate (UTP) (150 µM). Note the relative increase in ΔISC attributable to sodium blockade with amiloride (negative deflection) and decrease in forskolin-stimulated ΔISC at 24 hours under hypoxic conditions.
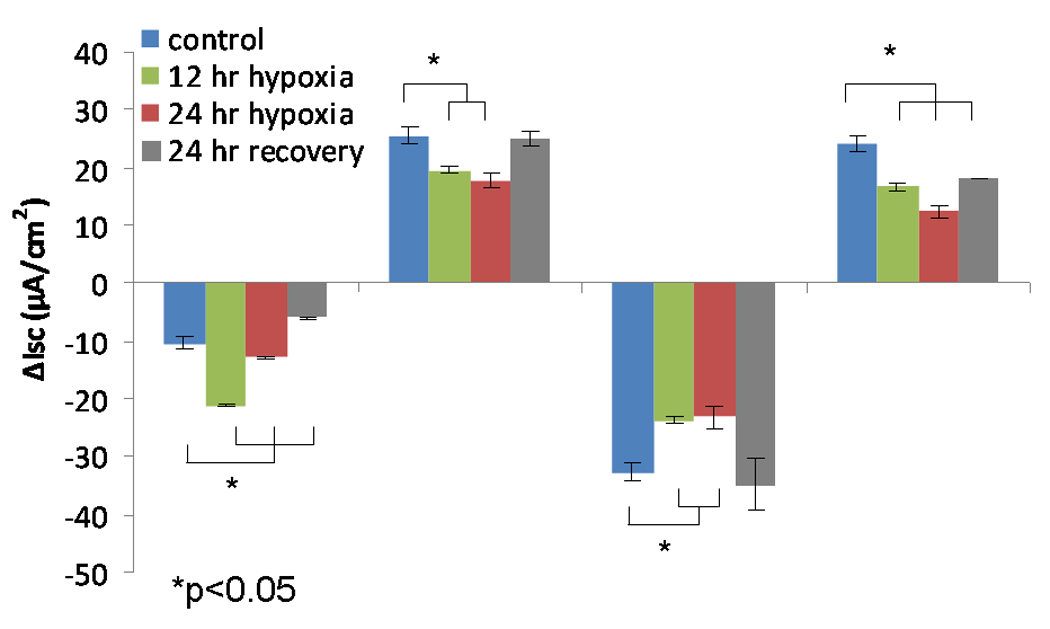
B. Change in short-circuit current (ΔISC) in human sinonasal epithelial cultures after 12 and 24 hours under hypoxic (1% O2) conditions and following 24 hour recovery period under physiologic conditions (21% O2). Forskolin-stimulated ΔISC was sensitive to hypoxic stress, and demonstrated significant reduction in CFTR-mediated Cl− transport (n ≥ 6 per condition). Decreased ΔISC from INH-172 blockade verified the contribution of CFTR to the ISC. Conversely, CaCC-mediated ΔISC (UTP-stimulated) was more susceptible to oxygen restriction compared to MNSE and demonstrated early inhibition at 12 hours. Sodium absorption (amiloride blockade) was significantly increased at 12 hours and returned back to baseline by 24 hours. HSNE demonstrated significant recovery of Cl− transport following 24 hours in a physiologic O2 (21%) environment.
