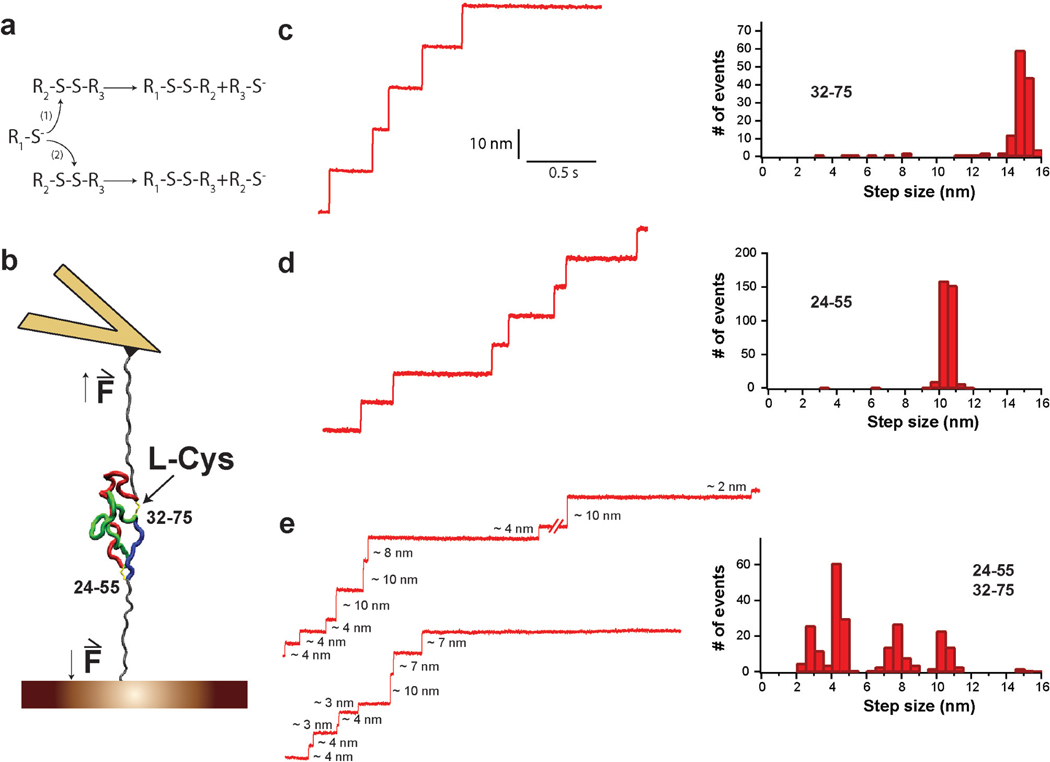
Figure 1. Increased complexity in the reduction of a protein with two disulfides.
a, Generic thiol-disulfide exchange reactions. b, Schematic experimental setup. Polyproteins containing several I27 modules with one or two disulfide bonds are unfolded by force-clamp AFM. For the sake of simplicity, only a single I27 module is shown. The depicted I27 module contains two disulfides linking positions 24–55 and 32–75; I272S-S. After mechanical unfolding, disulfides are rendered solvent accessible and can be cleaved by L-Cys. c, (Left) Experimental trace showing reduction events obtained for (I2732–75)8 polyprotein in the presence of L-Cys at 250 pN. (Right) The reduction events found for (I2732–75)8 are characterized by a single population of 14.5 nm steps. d, (Left) Experimental trace showing reduction events obtained for (I2724–55)8 polyprotein in the presence of L-Cys at 250 pN. (Right) The step sizes for the reduction events found for (I2724–55)8 fall into a single population centered at 10nm. e, (Left) Two experimental traces showing reduction events obtained for (I272S-S)4 polyprotein in the presence of L-Cys at 250 pN. (Right) The distribution of step sizes for the reduction events found for (I272S-S)4 is not a simple combination of the populations found for the proteins with only one disulfide.