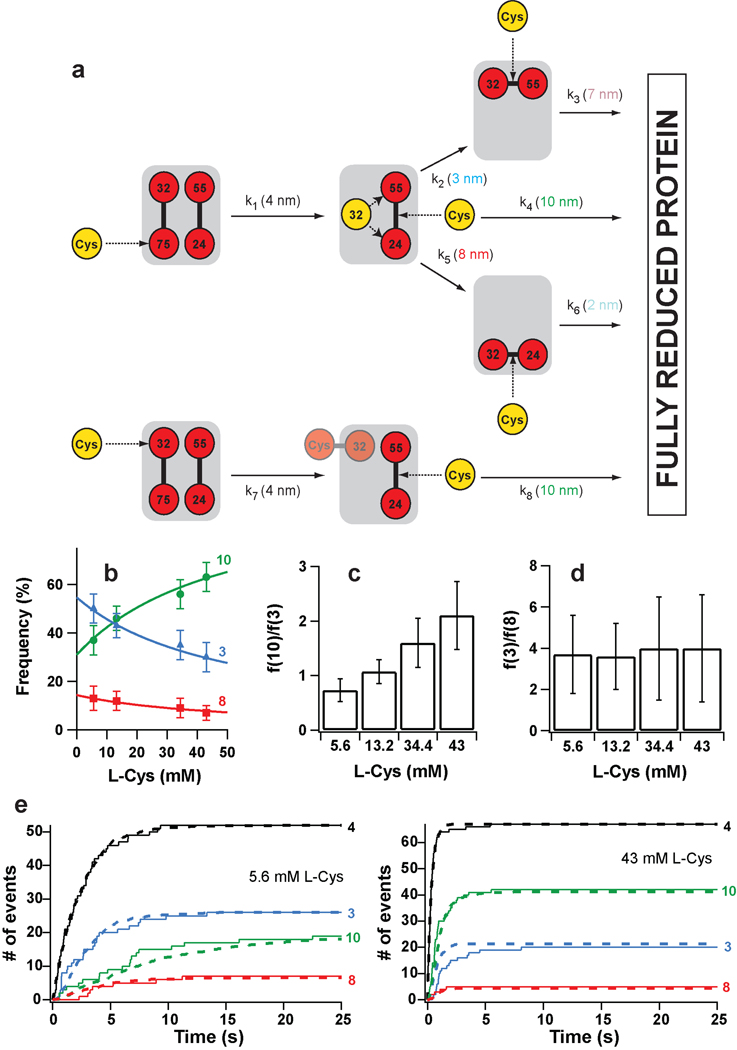
Figure 3. Kinetic model of complete protein reduction by L-Cys following the initial reduction of disulfide 32–75.
a, L-Cys can react with either sulfur in disulfide 32–75. (Top) Reaction at position 75 generates a free thiolate at position 32, which can then react intramolecularly with disulfide 24–55. This attack can happen at either sulfur atom, and competes with the intermolecular reaction with L-Cys. The intramolecular reactions generate new disulfides that can only be cleaved by L-Cys. (Bottom) If the initial reaction happens at position 32, the intramolecular reactions are blocked by the formation of a mixed disulfide between L-Cys and Cys32. In this case, a free thiol group is generated at position 75, but it is pulled away by force and can not participate in intramolecular exchanges with disulfide 24–55. The rate constants governing the reactions and their characteristic step sizes are indicated. b, The frequency of appearance of the step sizes depends on the concentration of L-Cys. The solid lines are the plots of Supplementary Equations S4–S6 using the rate constants derived from the downhill simplex method. c, With increasing concentrations of L-Cys, the relative frequency of appearance of the 10- and 3-nm increases. d, The relative frequency of the 3- and 8-nm steps is independent of L-Cys concentration. e, (Solid lines) Time course for the appearance of the different step sizes at two L-Cys concentrations. (Dashed lines) Theoretical curves obtained from the rate constants determined using the downhill simplex method. In panels b and e, traces are identified by the magnitude of the steps.