Table 2.
Optimization of the elimination protocol and formation of vinyl azide 4a under batch conditions (entries 1–7) and as a flow protocol (entry 8).
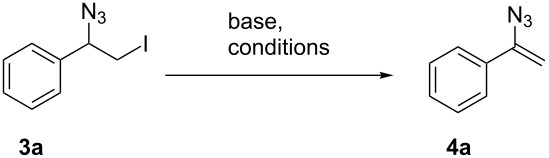 | |||||
| entry | base | solvent | T [°C] | t [h] | yielda |
| 1 | 1.5 equiv KOt-Bu | Et2O | rt | 2 | 95% |
| 2 | 1.5 equiv KOt-Bu | CH2Cl2 | rt | 2 | decomposition |
| 3 | 5 equiv K2CO3 | CH2Cl2 | rt | 18 | 5% |
| 4b | 5 equiv K2CO3 | CH2Cl2 | 60 | 18 | 23% |
| 5 | 2.5 equiv DIPEAc | DMF | 60 | 2 | 92% |
| 6 | 2 equiv DBU | CH2Cl2 | rt | 1.5 | 92% |
| 7 | 2 equiv PS–DBUd | CH2Cl2 | rt | 1.5 | 93% |
| 8e | 2 equiv PS–DBU | CH2Cl2 | rt | 0.04 mL/min | complete transformation |
aIsolated yields; bReaction was carried out in a microwave-compatible tube heated in an oil bath, cDIPEA = diisopropylethyl amine; dPS–DBU = polystyrene-bound 1,8-diaza-[5.4.0]bicyclo-7-undecene (8); eFlow process: Glass reactor (12 cm length and 8.5 mm internal diameter) filled with polymer 8 (0.5 g; theoretical loading = 1.15 mmol/g).
