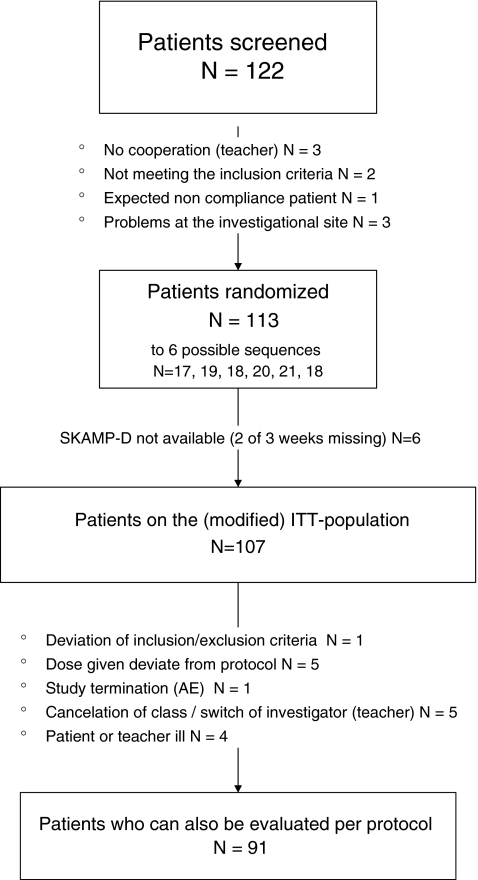
FIG. 1.
Flowchart of trial participants. *One patient showed signs of clinically significant laboratory values (raised transaminases and creatine kinase values) and was, thus, excluded early from the trial by the sponsor due to safety concerns. AE, adverse events; ITT, intent-to-treat; SKAMP-D, German version of the Swanson, Kotkin, Agler, M-Flynn and Pelham scale (rated by teachers).