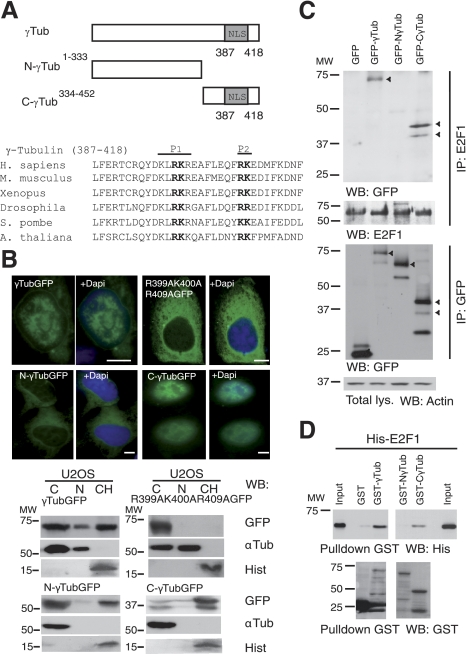
Figure 2.
γ-Tubulin C terminus contains a nuclear localization signal. A) Top panel: structure of wild-type γ-tubulin and various γ-tubulin constructs, depicting the nuclear localization signal (NLS). Bottom panel: bold type indicates the larger (P1) and the smaller (P2) basic patch of mutated amino acids in the γ-tubulin NLS. B) Top panels: immunofluorescence analysis of U2OS cells transfected with GFP-γ-tubulin (γTubGFP), γtubGFP-R399A-K400A-R409A (R399A-K400A-R409A), N-γtubGFP1–333 (N-γTubGFP), or C-γtubGFP334–452 (C-γTubGFP). Nuclei were detected using DAPI (blue). Scale bars = 10 μm. Bottom panels: cells (0.5×106) were analyzed biochemically, as summarized in Fig. 1A, and examined by WB (n=3–6). C) U2OS cells expressing the various GFP-γ-tubulin constructs, as indicated, were separately immunoprecipitated with first anti-E2F1 (top panels), and then the same lysate was immunoprecipitated a second time with anti-GFP antibodies (bottom panels) and analyzed by WB. Part of the lysate used for the immunoprecipitations was run as loading control (total lys.). Cellulose membranes containing immunoprecipitated E2F1 were first analyzed by WB with an anti-GFP antibody and reprobed with an anti-E2F1. Arrowheads indicate the various GFP-fused proteins. D) Purified full-length GST-γ-tubulin was incubated with His-E2F1, and the GST-γ-tubulin was subsequently retrieved by adsorption to glutathione-Sepharose 4B and examined by WB (n=3). Total amounts of loaded His-E2F1 were analyzed by WB (input).