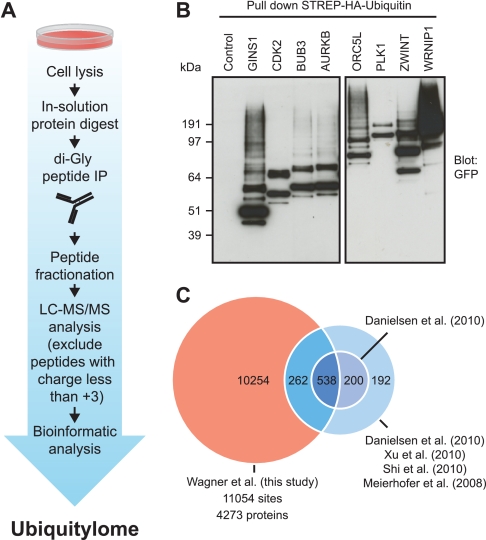
Fig. 1.
Proteome-wide mapping of endogenous ubiquitylation sites. A, Proteins from total cells lysates were digested into peptides using trypsin and ubiquitylated peptides were enriched with a monoclonal di-Gly-lysine-specific antibody. Immunoprecipitated peptides were fractionated and analyzed by mass spectrometry. Peptides with charge-state +1 and +2 were excluded from MS2 analysis to preferentially sequence ubiquitylated peptides. B, Verification of eight randomly selected proteins by Western blotting. Ubiquitylated proteins identified in our proteomic screen were affinity purified from cells expressing HA-tagged ubiquitin and ubiquitylation was confirmed by immunostaining with anti-GFP antibodies. C, Overlap of ubiquitylation sites identified in this study with sites combined from four previous MS-based ubiquitylation studies, or individually compared with the previous largest study, as indicated.