Abstract
Context:
Thyroid nodules are common in adults, but only a small fraction of them is malignant. Fine-needle aspiration (FNA) cytology provides a definitive diagnosis of benign or malignant disease in many cases, whereas about 25% of nodules are indeterminate, hindering most appropriate management.
Objective:
The objective of the investigation was to study the clinical utility of molecular testing of thyroid FNA samples with indeterminate cytology.
Design:
Residual material from 1056 consecutive thyroid FNA samples with indeterminate cytology was used for prospective molecular analysis that included the assessment of cell adequacy by a newly developed PCR assay and testing for a panel of mutations consisted of BRAF V600E, NRAS codon 61, HRAS codon 61, and KRAS codons 12/13 point mutations and RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements.
Results:
The collected material was adequate for molecular analysis in 967 samples (92%), which yielded 87 mutations including 19 BRAF, 62 RAS, 1 RET/PTC, and five PAX8/PPARγ. Four hundred seventy-nine patients who contributed 513 samples underwent surgery. In specific categories of indeterminate cytology, i.e. atypia of undetermined significance/follicular lesion of undetermined significance, follicular neoplasm/suspicious for a follicular neoplasm, and suspicious for malignant cells, the detection of any mutation conferred the risk of histologic malignancy of 88, 87, and 95%, respectively. The risk of cancer in mutation-negative nodules was 6, 14, and 28%, respectively. Of 6% of cancers in mutation-negative nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology, only 2.3% were invasive and 0.5% had extrathyroidal extension.
Conclusion:
Molecular analysis for a panel of mutations has significant diagnostic value for all categories of indeterminate cytology and can be helpful for more effective clinical management of these patients.
Thyroid cancer is the most common endocrine malignancy with an estimated occurrence of 45,000 cases in the United States in 2010. Its incidence continues to rise, and it is the fastest growing cancer in women in the United States. Typically, thyroid cancer occurs in thyroid nodules, which are estimated to affect more than 100 million people in the United States (1–3). However, most nodules are benign, and the rate of cancer in medically evaluated thyroid nodules ranges between 5 and 15% (4–8). A clinical challenge is to accurately diagnose cancer in these nodules and to avoid unnecessary thyroid surgery for benign disease.
Currently, the most common and reliable diagnostic tool for evaluation of thyroid nodules is fine-needle aspiration (FNA) cytology. FNA provides a definitive diagnosis of benign or malignant thyroid disease in most cases. However, in about 25% of nodules, FNA cytology cannot reliably exclude cancer, and such cases are placed in one of the indeterminate categories (2, 5, 9–11). By the current Bethesda System for Reporting Thyroid Cytopathology, the indeterminate categories include three specific cytological diagnoses: atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular or oncocytic (Hürthle cell) neoplasm/suspicious for follicular or oncocytic (Hürthle cell) neoplasm (FN/SFN), and suspicious for malignant cells (SMC), with a predicted probability of cancer of 5–15, 15–30, and 60–75%, respectively (12, 13). Because FNA is unable to provide a definitive diagnosis for these nodules, most patients with indeterminate cytology undergo diagnostic surgery to establish a histopathological diagnosis. However, only 10–40% of such surgically resected thyroid nodules will prove to be malignant (12, 14, 15). These unneeded operations, with their attendant expenses and risks, may be avoided if the FNA procedure could reliably establish the presurgical diagnosis of a benign nodule. Additionally, because the standard of care is to offer a second surgery to complete the thyroidectomy once diagnostic lobectomy confirms a cancer, a more optimal surgical management would be a single up-front total thyroidectomy that is offered when the diagnosis of cancer is established preoperatively. Furthermore, some genetic alterations (BRAF V600E) portend substantially worse prognosis and nodal metastasis (16–18) and thus extent of surgery may be impacted by the availability of this information preoperatively.
We and others have recently shown that testing for BRAF V600E (19) and for a panel of somatic mutations (20–22) is feasible in thyroid FNA material and provides helpful diagnostic information. The panel includes most common mutations including that collectively occur in approximately 70% of thyroid cancer, i.e. BRAF V600E, NRAS codon 61, HRAS codon 61, and KRAS codons 12/13 point mutations and RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements. The revised American Thyroid Association's management guidelines recommend to consider the mutational panel for nodules with indeterminate FNA cytology to help guide clinical management (23). However, the role of molecular testing has not been evaluated in a large cohort of patients with indeterminate cytology diagnoses, and its implications for optimal patient management are not well established. Here we report the results of a large prospective study that defines the diagnostic utility of mutational analysis, which is further enhanced by introducing a novel approach for assessing sample adequacy and propose a clinical management algorithm for patients with cytologically indeterminate thyroid nodules.
Materials and Methods
FNA samples
From April 2007 to April 2009, 1056 consecutive FNA samples from thyroid nodules with indeterminate cytology diagnoses established at the University of Pittsburgh Medical Center were prospectively tested for mutations in the clinical molecular diagnostic laboratory at the University of Pittsburgh Medical Center. Cytological diagnosis was established before molecular testing. Because at our institution the mutation analysis on all FNA samples with indeterminate cytology is standard of care, the molecular analysis did not require approval of the University of Pittsburgh Institutional Review Board. However, the retrospective analysis and correlation between the results of cytological evaluation, histological diagnosis, and molecular testing was performed using a protocol approved by the institutional review board. These samples were from 762 patients, including 294 patients who contributed multiple FNA samples collected from the same or different nodules, either at the same time or consecutively. For this study, each FNA sample was considered independently. Mutational analysis of 117 FNA samples from this cohort has been reported in our previous study (22). These samples were reanalyzed with the novel technique for assessment of sample adequacy as reported below. Routine FNA procedures were conducted under ultrasound guidance by a radiologist or an endocrinologist using a 23-, 25-, or 27-gauge needle. In most cases, three to four FNA passes were performed. Most of the aspirated sample from the first two passes was used to make direct cytology smears, whereas the residual material in the needle and the needle wash from both passes were placed into a tube containing 400 μl of nucleic acid preservative solution (Roche Molecular Biochemicals, Manheim, Germany) (22, 24). The tube was stored frozen at −20 C before molecular analysis.
Molecular analysis
Nucleic acid isolation
Total nucleic acids were isolated from FNA material collected into the preservative solution using MagNA Pure Compact instrument (Roche) and Nucleic Acid Isolation Kit I (Roche) according to the manufacturer's instructions.
Assessment of sample adequacy for molecular testing
The adequacy of samples for molecular analysis was assessed based on the general quantity and quality of isolated nucleic acids and the proportion of epithelial cells within the sample. The quantity and quality of isolated DNA and RNA was assessed by PCR amplification of the RAS and BRAF genes and GAPDH cDNA, respectively. It was considered satisfactory when the amplification cycle threshold (Ct) was less than 35 cycles.
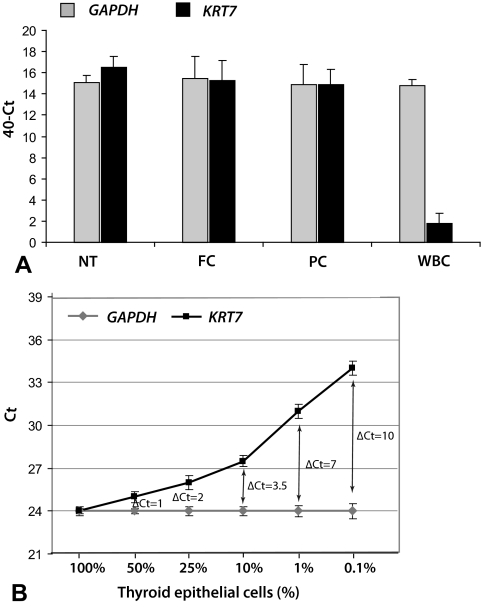
For the assessment of the proportion of epithelial cells within a FNA sample, a novel approach was developed. It was based on comparing the expression of the housekeeping gene GAPDH, which is uniformly expressed in all cells types, and the cytokeratin gene KRT7, which is expressed only in few distinct types of epithelial cells including thyroid cells. The expression of both genes was detected by real-time RT-PCR on ABI7500 (Supplemental Appendix, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The difference in the amplification between KRT7 and GAPDH of more than 3.5 cycles (i.e. CtKRT7 − CtGAPDH > 3.5) corresponded to approximately10% of thyroid epithelial cells within the sample and was used as a cutoff for sample adequacy for molecular analysis (Fig. 1).
Fig. 1.
Evaluation of FNA sample adequacy before mutational analysis using the expression of the GAPDH and KRT7 genes detected by RT-PCR. A, KRT7 and GAPDH were expressed at similar levels in normal thyroid tissues (NT) (n = 10), follicular carcinomas (FC) (n = 10), and papillary carcinomas (PC) (n = 40) (mean ΔCt = −0.41, where ΔCt is CtGAPDH − CtKRT7; P = 0.90). However, KRT7 was expressed at a significantly lower level in white blood cells (WBC), which are a frequent contaminant of thyroid FNA samples (mean ΔCt = 13; P < 0.001). B, Serial dilution of RNA from thyroid papillary carcinoma tissue in RNA derived from normal WBC demonstrated a linear decrease in KRT7 expression compared with stable GAPDH expression, with the ΔCt of 3.5 corresponding to approximately 10% of thyroid epithelial cells within the sample. The ΔCt of 3.5 was used as a cutoff for sample adequacy.
Mutational analysis
BRAF V600E, NRAS codon 61, HRAS codon 61, and KRAS codons 12 and 13 point mutations were detected using real-time LightCycler PCR (Roche) and fluorescence melting curve analysis (FMCA) as previously described (20). All samples that were positive for mutations on FMCA were confirmed by Sanger sequencing. RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements were detected by real-time RT-PCR with primers designed to flank the respective fusion point on ABI 7500 (Applied Biosystems, Foster City, CA) as previously described (20). As previously reported, the sensitivity of mutation detection was 10% of mutant alleles for BRAF and RAS and 1% of mutant alleles for RET/PTC and PAX8/PPARγ (20). In addition, for a more sensitive detection of BRAF V600E, samples that showed slight deviation of melting curve on post-PCR FMCA were tested with a more sensitive COamplification at Lower Denaturation-PCR (COLD-PCR) technique (Supplemental Appendix). Mutational analysis was performed by trained medical technologists in the clinical molecular diagnostic laboratory who were blinded to the results of cytological and final histopathological diagnoses.
Cytological and histological review
The initial histopathological diagnosis was established by a pathologist who was not blinded to the results of molecular testing. The results of cytological evaluation, histological diagnosis, and molecular analysis were reviewed retrospectively. At our institution, the transition to the Bethesda Reporting System of Thyroid Cytology took place in September 2008. Each cytology diagnosis established before September 2008 was reviewed and translated to match the currently used Bethesda terminology (12, 13). In those cases in which subsequent surgery was performed, the histological slides were reviewed to confirm the histopathological diagnosis of aspirated nodules.
Statistical analysis
Comparison of expression levels was performed using a paired two-tailed Student's t test on SAS version 9.2 software (SAS Institute Inc., Cary, NC). Calculations of specificity, sensitivity, positive predictive value, and negative predictive value were performed using MedCalc Statistical Software version 9.6 (Mariakerke, Belgium).
Results
Of 1056 consecutive FNA samples with indeterminate cytology, 50 were found to have insufficient amount of isolated nucleic acids and another 39 insufficient proportion of epithelial cells (CtKRT7 − CtGAPDH > 3.5) for mutational analysis. The remaining 967 FNA samples collected from 729 patients (92%) were subjected to mutational analysis. Among these samples, a cytological diagnosis of AUS/FLUS was established in 653 samples, FN/SFN in 247 samples, and SMC in 67 samples. Molecular analysis revealed 87 mutations including 19 BRAF V600E (15 by regular sensitivity PCR and FMCA and four by COLD-PCR), 47 NRAS codon 61, 12 HRAS codon 61, and three KRAS codons 12/13 point mutations, and one RET/PTC1 and five PAX8/PPARγ rearrangements.
To date, 479 patients underwent thyroidectomy, which provided a histopathological diagnosis for 513 FNA samples. Indications for surgery at our medical center include the cytological diagnosis of SMC and FN/SFN as well as AUS/FLUS cytology followed by either AUS/FLUS or higher cancer risk cytology on repeated FNA. Among the histologically confirmed 513 FNA samples, 61 RAS, 17 BRAF V600E, one RET/PTC, and four PAX8/PPARγ mutations were identified. The results of mutational analysis in each group of indeterminate cytology were correlated with the surgical pathology diagnosis.
AUS/FLUS group
This group included 653 FNA samples with cytological diagnosis of AUS/FLUS. They were collected from 439 patients, of whom 214 had either two nodules aspirated simultaneously and both diagnosed as AUS/FLUS, or had repeated FNA biopsies of a nodule yielding the diagnosis of AUS/FLUS. Additionally, 61 patients in this group had a repeated FNA biopsy of the same nodule that yielded the cytological diagnosis of FN/SFN or SMC, and 151 patients had a repeated FNA that yielded the cytological diagnosis of a benign nodule. At surgery, 218 patients (with 247 FNA) had the histopathological diagnosis of malignancy in 32 nodules (35 FNA) and benign disease in 184 nodules (212 FNA).
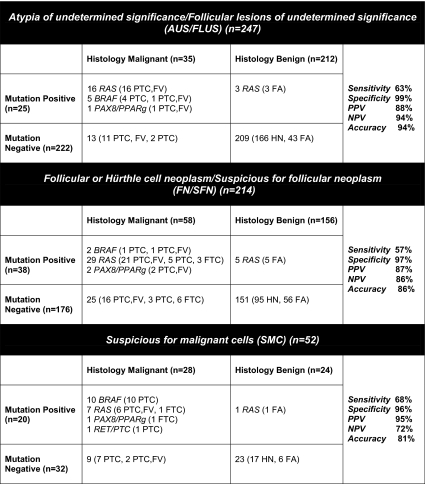
Mutations were identified in 25 FNA samples, including 19 RAS, five BRAF V600E, and one PAX8/PPARγ. The correlation between mutations and outcome is summarized in Fig. 2. Twenty-two mutation-positive FNA (88%) were from cancer nodules. Three false-positive nodules (all with RAS mutations) were follicular adenomas. Thirteen mutation-negative nodules (5.9%) were found to be malignant after surgery, providing a negative predictive value of 94% and specificity of 99%.
Fig. 2.
Correlation between the results of mutational analysis in FNA samples and outcome in specific groups of indeterminate cytology. PTC, Papillary thyroid carcinoma; PTC, FV, papillary thyroid carcinoma, follicular variant; FTC, follicular thyroid carcinoma; FA, follicular adenoma; HN, hyperplastic nodule.
Thirty-two cancer nodules in this group were sampled by 35 FNAs, including three nodules that were aspirated and tested twice, all yielding the identical result of molecular testing. These 32 cancers included 18 invasive papillary carcinomas (five classic type, one tall cell variant, and 12 follicular variant) and 14 noninvasive encapsulated follicular variants of papillary carcinoma. Six tumors showed extrathyroidal extension, and three of these had lymph node metastasis at surgery. Correlating each FNA sample with an outcome individually, mutations were identified in 22 of 35 cancer nodules (63%), including 14 of 19 invasive cancers, five of six cancers with extrathyroidal extension, and two of three cancers with lymph node involvement. Among the 13 mutation-negative cancers, eight were noninvasive encapsulated follicular variants of papillary carcinoma, four were encapsulated intrathyroidal cancers with invasive features (either tumor capsule invasion or vascular invasion), and one was a classic papillary carcinoma with extrathyroidal extension and lymph node metastasis. As a result, the risk of cancer in nodules with AUS/FLUS cytology and negative for mutations was 5.9%. Furthermore, the risk of intrathyroidal cancer with invasive features was 2.3%, and that of extrathyroidal spread was 0.5%.
FN/SFN group
This group included 247 FNA samples with the cytological diagnosis of FN/SFN, collected from 232 patients. To date, 210 patients (214 FNA samples) underwent surgery, yielding the histopathological diagnosis of malignancy in 58 aspirated nodules (27%). Thirty-eight mutations were identified in nodules that were resected, including 34 RAS mutations, two BRAF V600E, and two PAX8/PPARγ. All BRAF and PAX8/PPARγ-positive nodules were malignant. Of 34 FNAs positive for RAS mutations, 29 were from malignant nodules, including 26 papillary carcinomas and three follicular carcinomas, whereas five were from follicular adenomas (Fig. 2). Overall, 33 of 38 mutation-positive nodules (87%) were found to be malignant after surgery, whereas five (13%) were RAS-positive benign follicular adenomas. Among 176 mutation-negative samples, 151 (86%) were benign and 25 (14%) were malignant.
SMC group
Sixty-seven thyroid FNA samples collected from 58 patients were diagnosed as SMC by cytology. Of those, 51 patients (52 FNA samples) underwent surgery, yielding the histopathological diagnosis of malignancy in 28 nodules (54%) (Fig. 2). Twenty mutations were identified in nodules that were resected. Nineteen mutation-positive nodules (95%) were malignant, and among them 10 harbored BRAF V600E, seven RAS, one RET/PTC, and one PAX8/PPARγ. One RAS-positive nodule was a benign follicular adenoma. Among 32 mutation-negative nodules with SMC cytology, 23 (72%) were benign and nine (28%) were found to be malignant after surgery.
Table 1 summarizes the impact of mutational testing on cancer probability in thyroid nodules in each group of indeterminate cytology and provides correlation between specific mutation detected in a FNA sample and cancer risk.
Table 1.
Cancer probability in thyroid nodules with indeterminate cytology based on specific cytological diagnosis and results of molecular testing performed in FNA samples
| Cancer risk |
||||
|---|---|---|---|---|
| AUS/FLUS cytology (n = 247) | FN/SFN cytology (n = 214) | SMC cytology (n = 52) | All indeterminate cytology (n = 513) | |
| Cytology only | 14% | 27% | 54% | 24% |
| Any mutation identified | 88% | 87% | 95% | 89% |
| RAS | 84% | 85% | 88% | 85% |
| BRAF | 100% | 100% | 100% | 100% |
| PAX8/PPARγ | 100% | 100% | 100% | 100% |
| RET/PTC | na | na | 100% | 100% |
| No mutation identified | 5.9% | 14% | 28% | 11% |
na, Not applicable.
Correlation between molecular findings in FNA and surgical samples
For all 121 malignant tumors identified after surgery, histological review confirmed that cancer arose in the nodule that was aspirated and allowed selection of tissue to repeat the molecular testing. All mutations identified in FNA samples were confirmed in the corresponding surgically removed nodules. Among cancer nodules that were negative for mutations when tested in the FNA material, one BRAF V600E mutation and three RAS mutations were additionally detected in the surgically removed tumor tissue. This indicates that our approach for testing thyroid FNA samples detected 95% of all mutation occurring in these cancer nodules.
Discussion
The results of this large prospective study indicate that mutational testing using residual material obtained during routine FNA allows more accurate cancer risk stratification of thyroid nodules with indeterminate cytology, which can be used to offer definitive surgical care in the first surgical procedure.
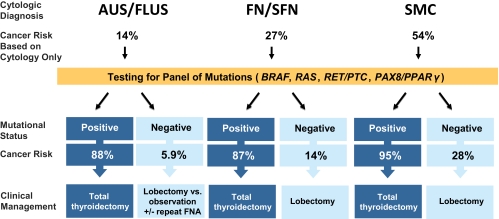
Histological correlation in a cohort of 513 cytologically indeterminate aspirates demonstrates that molecular testing has a high positive predictive value of 87–95% for predicting thyroid cancer. This risk exceeds the 50–75% risk of malignancy associated with a cytological diagnosis of SMC, for which current American Thyroid Association guidelines recommend total thyroidectomy (23). Because the presence of any mutation conveys an even greater cancer risk, it seems appropriate to suggest that any positive genetic test result in this panel should be considered an indication for total thyroidectomy in all categories of indeterminate cytology (Fig. 3). This approach eliminates the need for a common two-step approach, i.e. diagnostic lobectomy followed by completion thyroidectomy, for most patients with malignant nodules.
Fig. 3.
Proposed clinical algorithm for management of patients with cytologically indeterminate thyroid FNA applying the results of mutational analysis.
It is important to recognize that BRAF V600E, RET/PTC, and PAX8/PPARγ mutations were associated with malignancy in close to 100% of nodules in this study and other reported series (25). Only the RAS mutation has been found to be associated with a somewhat lower risk of thyroid malignancy, which was 85% in this study and 74–88% in other reported series (20, 21). The remaining RAS-positive cases were typically follicular adenomas without definitive features of malignancy identified histologically. Importantly, RAS is a potent oncogene and some indirect evidence exists, suggesting that it may promote malignant transformation and subsequent tumor dedifferentiation in thyroid cells (26–30). However, whether follicular adenomas are premalignant lesions and may progress to follicular carcinomas remains unknown. Therefore, in the specific case of RAS-positive lesions, informed discussion should include the discussion of the cancer risk and consideration of diagnostic lobectomy. In some satiations and taking into account the status of the opposite lobe, the sufficiently high cancer risk associated with this mutation may justify total thyroidectomy as the appropriate first-line recommendation.
With respect of the mutation-positive cytological samples and their histopathological correlation, it is important to consider that in most cases pathologists were aware of the results of molecular analysis, which could influence the diagnostic process in several ways. First, the surgically removed mutation-positive thyroid nodules were more likely to undergo more exhaustive gross, microscopic, and immunohistochemical analyses to identify malignant features. Second, thyroid nodules positive for a clonal mutation had a higher chance to be diagnosed as a follicular adenoma rather than a hyperplastic (goiter) nodule, even if the nodule had significant colloid content. This is because a nodule composed of cells carrying the same genetic mutation has a monoclonal origin, i.e. is biologically a neoplasm rather than a hyperplastic process, even in light of ambiguous histopathological features.
Negative predictive value of molecular testing varies between different groups of indeterminate cytology. In this study, cancer risk in mutation-negative nodules with FN/SFN and SMC cytology was 14 and 28%, respectively. This moderate cancer risk is virtually identical to those of FN/SFN cytology without molecular testing (15–30%) (12, 13), for which current American Thyroid Association guidelines recommend diagnostic lobectomy with consideration of total thyroidectomy when other appropriate factors are present (23). Therefore, when no such factors exist, diagnostic lobectomy appears to be justified as initial surgical intervention for mutation-negative nodules with FN/SFN and SMC cytology. The appropriate approach to nodules with AUS/FLUS cytology that are negative for mutations is less clear. These nodules have a cancer risk of only 6%, with a risk of cancer extending outside the thyroid gland of less than 1%. Because these risks are low, conservative management with ultrasound follow-up and repeat FNA in lieu of definitive surgical management may be reasonable in appropriately selected patients.
In this study, the additional value of molecular analysis in refining the cancer risk of specific cytological diagnoses was calculated using each individual encounter, i.e. a single FNA sample. However, in clinical practice, patients with indeterminate cytology, particularly those with the diagnosis of AUS/FLUS, frequently have one or more repeated FNA procedures performed on the same nodule. The repeated FNA can yield a different cytological diagnosis, which may refine clinical management in the absence of molecular testing.
Our results indicate that collection of residual aspirated material for molecular testing during routine FNA procedure directly into nucleic acid preservative solution is simple and effective and does not prolong the medical procedure. The collected material was sufficient for mutation detection in more than 90% of cases based on the monitoring of adequacy and cellular composition using a newly developed molecular assay. This approach allows us to capture 95% of mutations using thyroid FNA samples, whereas several additional mutations were detected in these nodules using surgically removed tissue. The discrepancy between mutation detection in FNA and corresponding tissue samples, also observed by Cantara et al. (21), can be explained by sampling only several areas of the nodule by FNA and by using a small portion of the collected FNA material for molecular analysis.
This single-institution, prospective study reports the largest known cohort of patients with all categories of indeterminate thyroid cytology classified according to the standardized Bethesda diagnostic criteria (12, 13). Data from this study and two smaller reported series of patients with indeterminate cytology (20, 21) suggest that the results of mutational testing are reproducible when the analysis is performed in an experienced diagnostic molecular laboratory. The replication of our findings by other groups in an even larger series of thyroid nodules with indeterminate cytology will help to further refine the proposed management of patients with thyroid nodules based on the combination of cytological evaluation and molecular analysis.
Acknowledgments
We thank Drs. David Cooper and R. Michal Tuttle for their helpful comments on the manuscript.
This work was supported in part by the National Institutes of Health Grant CA88041 and by the Richard A. and Leslie A. Snow Fund for Thyroid Cancer Research, Pittsburgh, Pennsylvania.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUS/FLUS
- Atypia of undetermined significance/follicular lesion of undetermined significance
- Ct
- cycle threshold
- FMCA
- fluorescence melting curve analysis
- FNA
- fine-needle aspiration
- FN/SFN
- follicular or oncocytic (Hürthle cell) neoplasm/suspicious for follicular or oncocytic neoplasm
- SMC
- suspicious for malignant cells.
References
- 1. Mazzaferri EL. 1992. Thyroid cancer in thyroid nodules: finding a needle in the haystack. Am J Med 93:359–362 [DOI] [PubMed] [Google Scholar]
- 2. Gharib H. 2004. Changing trends in thyroid practice: understanding nodular thyroid disease. Endocr Pract 10:31–39 [DOI] [PubMed] [Google Scholar]
- 3. Rojeski MT, Gharib H. 1985. Nodular thyroid disease. Evaluation and management. N Engl J Med 313:428–436 [DOI] [PubMed] [Google Scholar]
- 4. Mazzaferri EL, de los Santos ET, Rofagha-Keyhani S. 1988. Solitary thyroid nodule: diagnosis and management. Med Clin North Am 72:1177–1211 [DOI] [PubMed] [Google Scholar]
- 5. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. 2006. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16:109–142 [DOI] [PubMed] [Google Scholar]
- 6. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD, Jr, Larsen PR, Marqusee E, Alexander EK. 2006. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 91:3411–3417 [DOI] [PubMed] [Google Scholar]
- 7. Kim DL, Song KH, Kim SK. 2008. High prevalence of carcinoma in ultrasonography-guided fine needle aspiration cytology of thyroid nodules. Endocr J 55:135–142 [DOI] [PubMed] [Google Scholar]
- 8. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. 2002. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 87:1941–1946 [DOI] [PubMed] [Google Scholar]
- 9. Greaves TS, Olvera M, Florentine BD, Raza AS, Cobb CJ, Tsao-Wei DD, Groshen S, Singer P, Lopresti J, Martin SE. 2000. Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer 90:335–341 [PubMed] [Google Scholar]
- 10. Sclabas GM, Staerkel GA, Shapiro SE, Fornage BD, Sherman SI, Vassillopoulou-Sellin R, Lee JE, Evans DB. 2003. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am J Surg 186:702–709; discussion 709–710 [DOI] [PubMed] [Google Scholar]
- 11. Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, Moore FD, Jr, Kim BW, Nosé V, Marqusee E, Larsen PR, Alexander EK. 2007. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 111:508–516 [DOI] [PubMed] [Google Scholar]
- 12. Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, Vielh P, DeMay RM, Sidawy MK, Frable WJ. 2008. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 36:425–437 [DOI] [PubMed] [Google Scholar]
- 13. Ali SZ, Cibas ES. 2010. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer; [DOI] [PubMed] [Google Scholar]
- 14. Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. 2002. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol 26:41–44 [DOI] [PubMed] [Google Scholar]
- 15. Mazzaferri EL. 1993. Management of a solitary thyroid nodule. N Engl J Med 328:553–559 [DOI] [PubMed] [Google Scholar]
- 16. Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, Kim M, Tufaro A, Ladenson P, Zeiger M, Tufano R. 2009. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol 27:2977–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. 2008. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93:3943–3949 [DOI] [PubMed] [Google Scholar]
- 18. Yip L, Nikiforova MN, Carty SE, Yim JH, Stang MT, Tublin MJ, Lebeau SO, Hodak SP, Ogilvie JB, Nikiforov YE. 2009. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery 146:1215–1223 [DOI] [PubMed] [Google Scholar]
- 19. Kim SW, Lee JI, Kim JW, Ki CS, Oh YL, Choi YL, Shin JH, Kim HK, Jang HW, Chung JH. 2010. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab 95:3693–3700 [DOI] [PubMed] [Google Scholar]
- 20. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. 2009. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 21. Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, Di Santo A, Caruso G, Carli AF, Brilli L, Montanaro A, Pacini F. 2010. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab 95:1365–1369 [DOI] [PubMed] [Google Scholar]
- 22. Ohori NP, Nikiforova MN, Schoedel KE, LeBeau SO, Hodak SP, Seethala RR, Carty SE, Ogilvie JB, Yip L, Nikiforov YE. 2010. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.” Cancer Cytopathol 118:17–23 [DOI] [PubMed] [Google Scholar]
- 23. Cooper DS, Doherty GM, Haugen BR, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 24. Nikiforov YE. 2009. Papillary carcinoma. In: Nikiforov YE, Biddinger PW, Thompson LDR. eds. Diagnostic pathology and molecular genetics of the thyroid. Baltimore: Lippincott, Williams, Wilkins; 160–213 [Google Scholar]
- 25. Nikiforova MN, Nikiforov YE. 2009. Molecular diagnostics and predictors in thyroid cancer. Thyroid 19:1351–1361 [DOI] [PubMed] [Google Scholar]
- 26. Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. 2003. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 120:71–77 [DOI] [PubMed] [Google Scholar]
- 27. Fagin JA. 2002. Minireview: branded from the start-distinct oncogenic initiating events may determine tumor fate in the thyroid. Mol Endocrinol 16:903–911 [DOI] [PubMed] [Google Scholar]
- 28. Basolo F, Pisaturo F, Pollina LE, Fontanini G, Elisei R, Molinaro E, Iacconi P, Miccoli P, Pacini F. 2000. N-ras mutation in poorly differentiated thyroid carcinomas: correlation with bone metastases and inverse correlation to thyroglobulin expression. Thyroid 10:19–23 [DOI] [PubMed] [Google Scholar]
- 29. Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, Wu R, Carcangiu ML, Costa J, Tallini G. 2003. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 21:3226–3235 [DOI] [PubMed] [Google Scholar]
- 30. Burns JS, Blaydes JP, Wright PA, Lemoine L, Bond JA, Williams ED, Wynford-Thomas D. 1992. Stepwise transformation of primary thyroid epithelial cells by a mutant Ha-ras oncogene: an in vitro model of tumor progression. Mol Carcinog 6:129–139 [DOI] [PubMed] [Google Scholar]