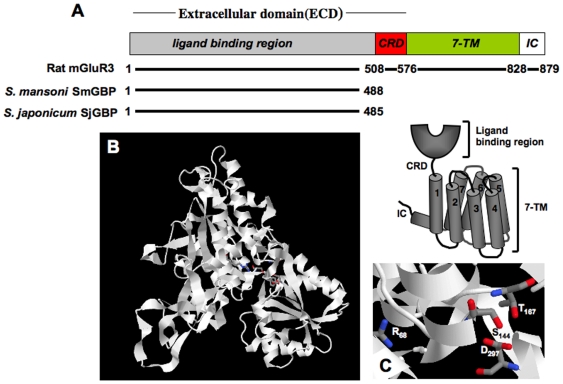
Figure 1. SmGBP is a C-terminally truncated metabotropic glutamate receptor.
(A) Predicted structure of Schistosoma mansoni SmGBP (Accession # HM483390) and a closely related Schistosoma japonicum homologue SjGBP (Sjp 0088520.1) compared to the crystal structure of rat mGluR3 (group II subtype 3) receptor (2e4u). The cartoon illustrates the expected domain architecture of metabotropic glutamate receptors (mGluR). The extracellular binding domain (ECD) includes a conserved Venus Flytrap module, which contains the glutamate binding region and is linked to the heptahelical membrane-anchoring segment (7-TM) by a cysteine-rich domain (CRD). The 7-TM segment is followed by an intracellular C-terminal domain (IC) of variable length. The amino acid positions of the various domains in mGluR3 are marked. Compared to the rat receptor, the schistosome SmGBP and SjGBP sequences contain the ligand binding region but lack the remaining cysteine-rich, membrane spanning and intracellular domains. (B) Hypothetical homology model of SmGBP showing the characteristic bilobed Venus Flytrap architecture of the mGluR ligand binding domain. The model was generated from an alignment with the crystal structure of rat mGluR3 (2e4u) using the modeling tools available at MODBASE [37]. (C) Predicted ligand binding residues of SmGBP were identified by comparison with known glutamate binding sites of mGluR3 [38]. Binding sites that are conserved in SmGBP include (corresponding mGluR3 position): Arg68 (Arg68), Ser144 (Ser151), Thr167 (Thr174), Tyr215 (Tyr222) and Asp297 (Asp301).