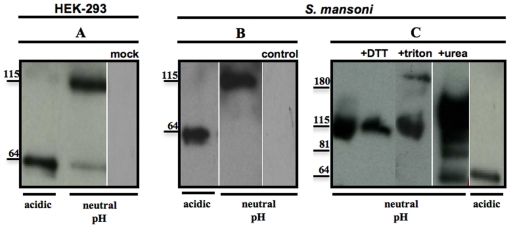
Figure 3. Immunoprecipitation (IP) of recombinant and native SmGBP.
Recombinant SmGBP was immunoprecipitated from transfected HEK-293 cell lysates (A) and native SmGBP was immunoprecipitated from a preparation of solubilized S. mansoni membrane proteins (B, C), as described in the text. In both cases, the IP was performed with anti-SmGBP antibody that was covalently linked to agarose beads. The protein was eluted from the beads at pH 2.8 (acidic) and the eluate was subsequently neutralized to pH 7.4 (neutral pH). Aliquots of the acidic and neutralized IP eluate were immunoblotted with peptide purified anti-SmGBP antibody. Under acidic conditions the antibody identified one major band at ≈ 60 kDa, whereas upon neutralization the antibody recognized a second band at ≈ 115 kDa both in transfected HEK-293 cells and S. mansoni. No bands were seen in the negative controls, either HEK-293 cells transfected with empty plasmid (mock) or blots probed with preimmune serum (control). (C) Native SmGBP was immunoprecipitated as above and treated with 0.1 M DTT, 1% triton X-100, 6 M urea or acid conditions (pH 2.8) prior to western blotting with anti-SmGBP antibody. The positions of relevant molecular weight markers are shown.