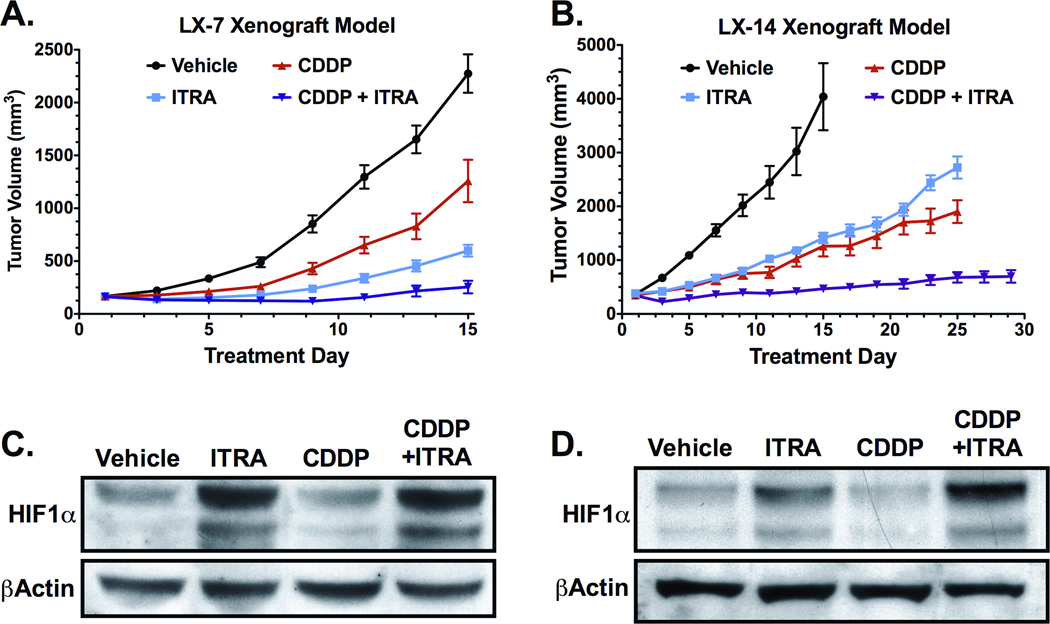
Figure 4. Itraconazole inhibits growth of NSCLC primary xenografts, with itraconazole treated tumors demonstrating increased levels of HIF1α.
(A) Mice bearing established LX-7 tumors were treated with vehicle (n=9), oral itraconazole 100 mg/kg bid (ITRA, n=9), cisplatin 4 mg/kg q7d i.p. (CDDP, n=9), or combination itraconazole and cisplatin (ITRA+CDDP, n=8). Mean tumor volume and SEM are reported for each treatment group. (B) Mice bearing established LX-14 tumors were treated with vehicle (n=6), oral itraconazole 100 mg/kg bid (ITRA, n=7), cisplatin 4 mg/kg q7d i.p. (CDDP, n=6), or combination itraconazole and cisplatin (ITRA+CDDP, n=7). Mean tumor volume and SEM are reported for each treatment group. (C) Tumor lysates from LX-7 xenografts were generated from mice treated for 14-days with vehicle (n=4), oral itraconazole (ITRA, n=4), i.p. cisplatin (CDDP, n=4), or combination of itraconazole and cisplatin (ITRA+CDDP, n=4). Pooled lysates were probed for HIF1α. (D) HIF1α immunoblot on pooled tumor lysates from LX-14 xenografts treated for 14-days with vehicle (n=4), oral itraconazole (ITRA, n=4), i.p. cisplatin (CDDP, n=4), or combination of itraconazole and cisplatin (ITRA+CDDP, n=3).