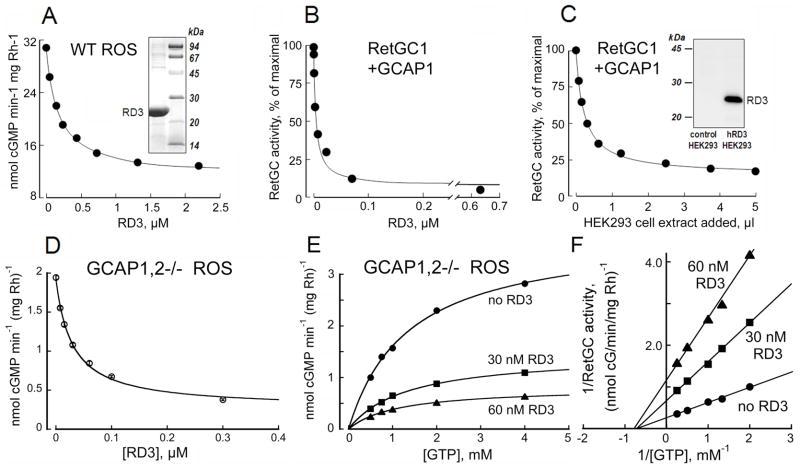
Fig. 1.
RD3 inhibits RetGC activity at submicromolar concentrations. A. Wild type mouse ROS were titrated with human recombinant RD3 in the presence of 1 mM free Mg2+ and 2 mM EGTA. Inset, Coomassie Brilliant Blue R250 - stained gel of human RD3 isolated from E. coli (left lane) and molecular weight standards (right lane). B. Human recombinant RetGC1 activated by 1.5 μM GCAP1 in the presence of 1 mM free Mg2+ and 2 mM EGTA was assayed at different concentrations of the human recombinant RD3 expressed and purified from E. coli. C. Human recombinant RetGC1 was activated by 1.5 μM bovine GCAP1 in the presence of 1 mM free Mg2+ and 2 mM EGTA and titrated with protein extracts from HEK293 cells either expressing or not expressing RD3. To exclude the possibility of a non-specific effect of different total protein concentrations, the total amount of protein was equilibrated with a control protein extract in every assay mixture. Inset, immunoblotting of HEK293 cell extracts probed with anti-RD3 antibody 497, left lane - non-transfected cells; right lane – RD3 plasmid-transfected cells (notice that there is no endogenous RD3 expression in the non-transfected cells). The data in A–D are fitted by the equation, a = (amax − amin)/(1+[RD3]/IC50) + amin; where amax and amin are the maximal and minimal activity of guanylyl cyclase in the experiment, respectively, and the IC50 is the concentration of RD3 producing 50% inhibition. D–F. The effect of RD3 on guanylyl cyclase catalytic activity in ROS fractions measured in the absence of GCAPs. The RetGC activity in ROS fraction from GCAP1,2−/− mouse retinas (24) was titrated with the purified E. coli-expressed RD3 in the presence of saturating 10 mM Mg2+ and 2 mM EGTA; GTP concentration in D was 1 mM and in E–F varied as indicated. E. Michaelis plot of the non-stimulated RetGC catalytic activity in the absence (●) or in the presence of 30 nM (■) or 60 nM (▲) purified recombinant RD3, representative from three similar independent experiments. F. Lineweaver-Burke plot for data from panel E illustrates a respective ~2.5-fold and 5-fold suppression of the Vmax by 30 nM and 60 nM RD3 without a major effect on KmGTP. The Ki calculated using the equation for a noncompetitive inhibition, Vi=Vmax(1+[RD3]/Ki)−1, from three independent experiments was 19 nM ± 7 SD. The activity in assays containing retinal membranes is presented per rhodopsin content, in membranes expressing recombinant RetGC it is normalized by the maximal activity for each series of the membrane preparations.