Fig. 4.
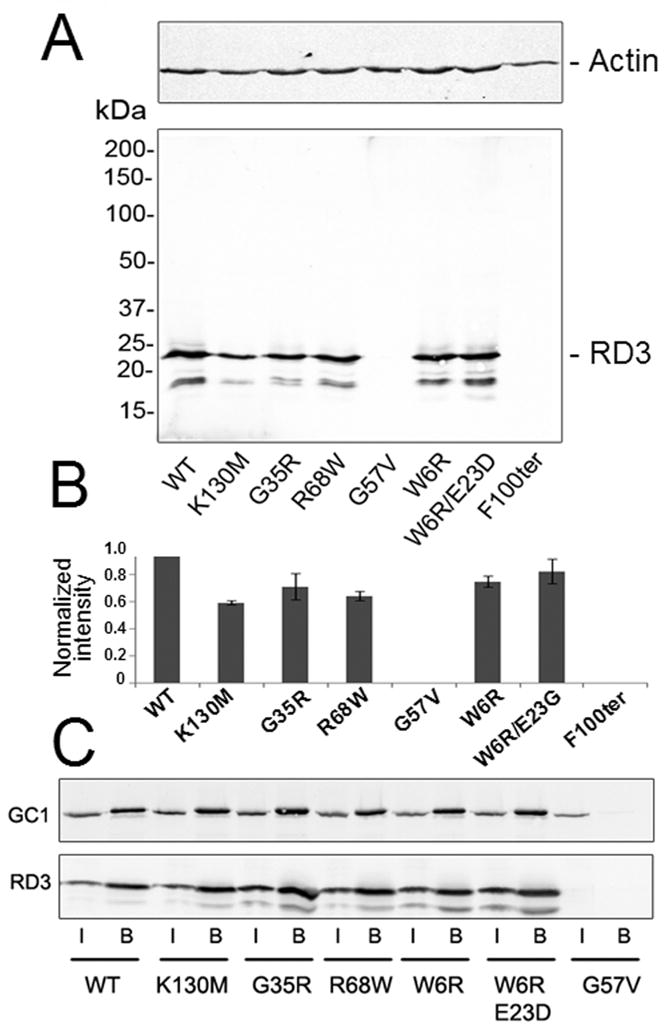
Mutations found in human patients with retinal diseases (15) affect RD3 expression in HEK293 cells. A. Expression of RD3 mutants in HEK293 cells. Human wild type and mutant RD3 tagged with the 1D4 peptide at the C-terminus were detected by immunoblotting as described in Experimental Procedures. Actin immunostaining (upper panel) was used as a protein load control. B. The average band intensities of the mutant RD3 proteins relative to WT RD3 band were determined from 3 independent experiments described in panel A (mean ± SD). C. Co-IP experiment. RetGC1 and RD3 were co-expressed as described in Experimental procedures and co-IP using Sepharose-coupled anti-RD3 9D12 antibody. The samples applied to the column (marked “I” for “input”) and the eluted fractions (marked “B” for “bound”) were analyzed by immunoblotting probed with anti-RetGC1 (GC-8A5; upper panel) and anti-RD3 (RD3-9D12; lower panel) antibodies. The G57V RD3 mutant that fails to express in HEK293 cells was used as a control to confirm that RetGC1 does not bind nonspecifically to the 9D12 antibody-coupled Sepharose. The lower band on the blot is specific for RD3-transfected cells and is most likely a partially proteolyzed RD3 polypeptide.
