Fig. 5.
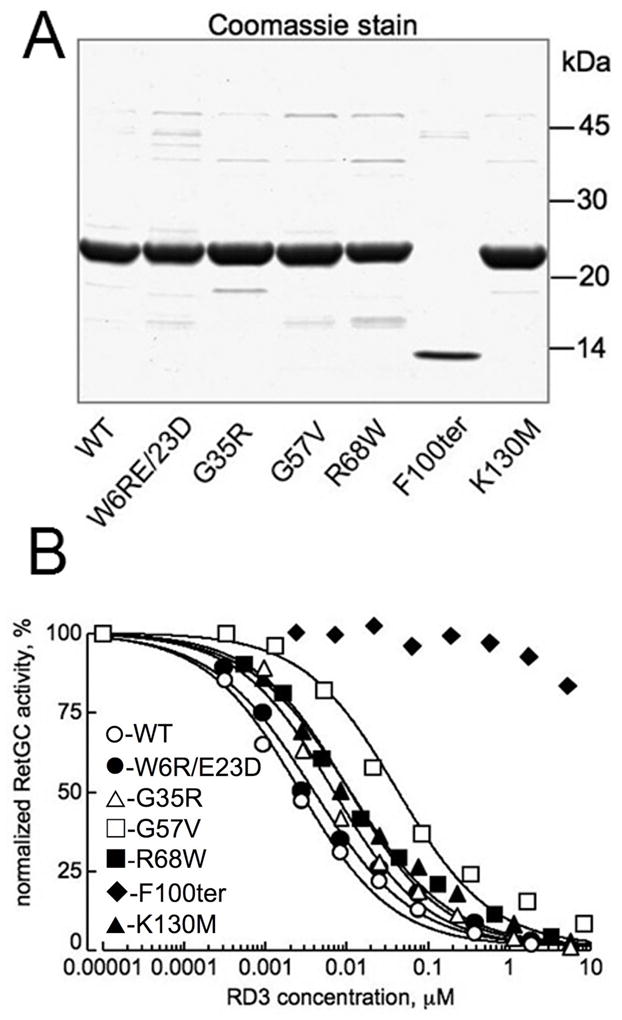
Effect of mutations in RD3 found in human patients with retinal diseases on RetGC activity in vitro. A. Wild type and mutant RD3 (15) expressed in E. coli were stained by Coomassie Brilliant Blue R-250 after SDS PAGE, 15% gel. For the use in subsequent RetGC assays, RD3 concentration in each case was that of the main band. B. Inhibition of RetGC1 activity by the E. coli - expressed RD3 mutants. RetGC activity in independent assays was normalized per activity measured in the absence of RD3. Recombinant RetGC1 expressed in HEK293 cells was reconstituted with 1.5 μM GCAP1 at indicated concentrations of RD3 (○) or its mutants: W6R/E23D (●), G35R (△), G57V (□), R68W (■), K130M (▲), and F100ter (◆). The IC50 values (mean ± SE, n) for the inhibition were 4.6 ± 1.3 nM, 5 (WT); 6.4 ± 2 nM, 3 (W6R/E23D); 7.9 ± 1 nM, 3 (G35R); 68 ± 13 nM, 3 (G57V); 17.3 ± 3.8 nM, 3 (R68W); and 14.3 ± 2.4 nM, 3 (K130M); the IC50 for the F100ter was ≫10 μM. The difference in the IC50 values from the wild type was significant (p in unpaired Student t-test assuming equal variance) for the G57V (<0.001), F100ter (<0.0001), R68W (0.012), and K130M (0.014) mutants.
