Figure 4.
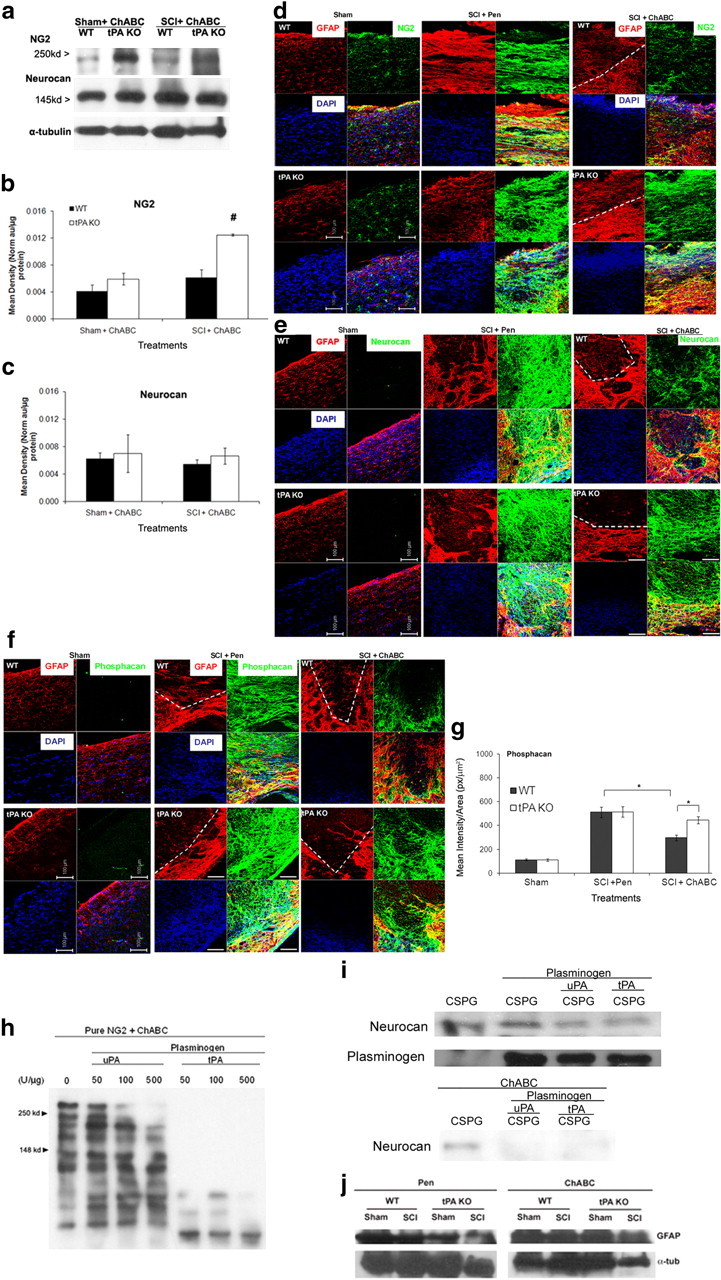
In the absence of tPA/plasmin system, NG2 and phosphacan core protein clearance is attenuated after ChABC cleavage in vivo. a, Representative immunoblots of NG2 and neurocan protein expressions in WT and tPA KO sham and 14 d SCI homogenates incubated with ChABC for 3 h at 37°C and probed with rabbit anti-NG2 and mouse anti-Neurocan antibodies. Protein expression could not be evaluated after Pen treatment in vitro using Western blotting since the presence of GAG chains resulted in a nondistinct protein band, data not shown. α-Tubulin was used as a loading control. b, c, Protein bands were quantified by densitometry in ImageJ and data normalized against α-tubulin loading control and total protein levels. One-way ANOVA was used to compare all groups. Significant ANOVA was followed by post hoc Holm–Sidak test. Significant post hoc differences at #p < 0.001 (n = 3). d–f, 14 d sham or contusion-injured WT and tPA KO mice received Pen or ChABC injection (50 U/ml × 1 μl). Two days later, spinal cords were isolated and perfused with PFA, and 18 μm sagittal sections were prepared. Spinal cord sections were then triple-stained for GFAP (red) and DAPI (blue, nuclear marker), and various CSPG protein antigens in green: NG2 (Millipore Bioscience Research Reagents) (b), phosphacan (3F8; DSHB) (d), and neurocan (1F6; DSHB) (f), and images were captured with a Zeiss confocal microscope using LSM 510 Meta Software. Dashed line indicates border of injury region. Images are representative of 3–4 biological replicates per group. Scale bar, 100 μm. g, ImageJ software (NIH) was used to calculate mean intensity/area for each group around the injury border (identified by GFAP dead and reactive regions). A t test was used to compare within each treatment and one-way ANOVA to compare within genotype (n = 3–4). Significant ANOVA values were followed by post hoc Holm–Sidak test. Brackets with asterisks indicate significance due to t test or post hoc analyses with a minimum of p < 0.05. h, Western blot of NG2 protein (gift from Dr. Joel Levine) incubated with ChABC and treated with recombinant plasminogen and different concentrations of uPA or tPA for 3 h at 37°C, followed by incubation with rabbit anti-NG2 antibody. ChABC cleaved NG2 protein can be seen at 250 and 148 kDa (arrowheads). i, Western blot of pure CSPG protein (CC117; Millipore Bioscience Research Reagents) incubated without (top blot) or with ChABC (bottom blot), pure plasminogen, uPA, or tPA at 500 U/μg and probed for neurocan protein (1F6, DSHB). Plasminogen protein levels (anti-mouse plasminogen 1:1000, Millipore) were also assessed (middle blot). j, Western blot of sham or SCI protein extracts from WT and tPA KO animals treated with Pen or ChABC. The blots were probed with GFAP (Abcam), Iba1 (data not shown), and α-tubulin.
