Fig. 12. DMS inhibits SOCE in a calcium-independent manner.
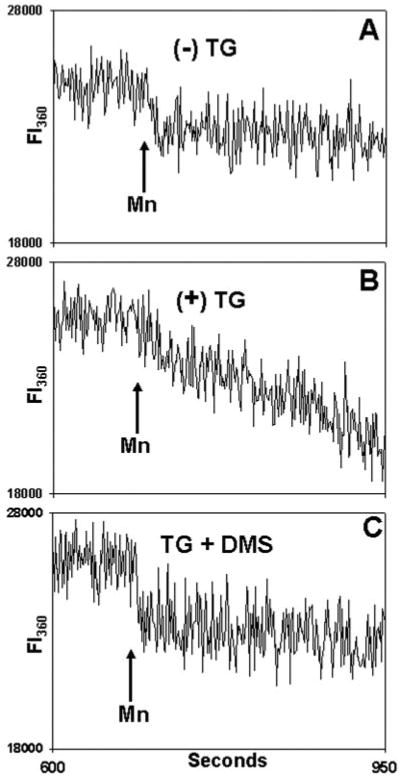
Fura-loaded PMN were incubated in 20 μm BAPTA for 9 min at room temperature. A, cells were exposed to vehicle only (Me2SO) at 30 s. Samples were then observed in the cuvette for 10 min. At that time, a mixture of 500 μm Mn2+ and 1.5 mm Ca2+ was added where indicated to quench Fura. In B, the cells were exposed to TG (500 nm) at 30 s. Again, 500 μm Mn2+ and 1.5 mm Ca2+ were added at the time indicated. In C, cells were pretreated for 1 min with 10 μm DMS and then exposed to TG (500 nm) at 30 s. Again, after allowing 10 min for store depletion, Mn2+ and Ca2+ were added. Fluorescent intensity (FI) was monitored at both 340/380 nm (not shown) and at 360 nm, as shown (FI360). No 340/380 nm [Ca2+]i release transient was seen at any time (data not shown). On Mn2+ addition, a small initial drop in total fluorescence at 360 nm is noted due to quenching of Fura in the media, but no Mn2+ entry into the cells was detected in the absence of prior store depletion (A). In contrast, prior TG treatment (B) caused rapid Mn2+ entry. TG-dependent Mn2+ entry was blocked by prior treatment with DMS (C). Linear curve-fitting (SigmaPlot) was then performed on the FI360 after Mn2+ addition (n = 3–4 independent experiments per condition). This revealed a mean slope of −2.3 ± 0.6 units/s after treatment with vehicle only. The FI360 slope decreased to −14.9 ± 0.9 units/s in TG-treated cells but was returned to unstimulated levels (−3.1 ± 0.4 units/s) by DMS pretreatment (p < 0.01, analysis of variance/Tukey's test). The data show that DMS inhibits the calcium entry response to TG store depletion in a [Ca2+]i -independent fashion.
