Figure 5.
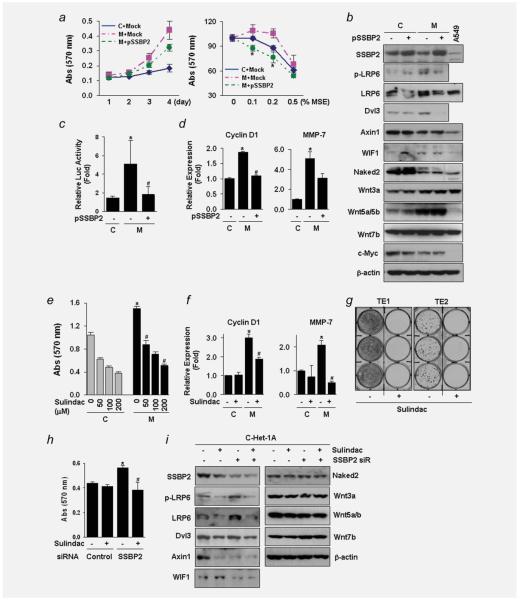
Suppression of the Wnt/β-catenin signaling by SSBP2. (a) Cell growth (left) and cellular sensitivity to MSE treatment (right) were measured by the MTT assay after incubation of cells (passage +31) transfected with pSSBP2 or empty vector control (Mock) for indicated time. MSE was treated for 48 hr. The results were expressed as absorbance at 570 nm. Values indicate means ± SD. *p < 0.05 compared to the Mock transfected-MSE-Het-1A cells (t-test). C, control-Het-1A cells; M, MSE-Het-1A cells; S, SSE-Het-1A cells. Expression of E-cadherin, an epithelial marker, was not changed, but N-cadherin level, a mesenchymal marker, was downregulated by SSBP2 expression in the MSE-Het-1A cells (data not shown). (b) Expression of proteins involved in the Wnt signaling was examined by western blotting in the control- and MSE-Het-1A cells (passage +31) with or without transfection of pSSBP2. Total cell lysates (30 μg/lane) were extracted run on 4–12% polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were then blotted for the indicated Wnt proteins. β-actin was used as a loading control. A protein lysate from the A549 lung cancer cell line (15 μg/lane) was used to compare expression level of each protein with that in Het-1A cells. No difference in p-LRP6 and Wnt7b expression was observed between the control- and MSE-Het-1A cells. (c) Luciferase assay after transfection of TOPflash or FOPflash reporter constructs into the control- and MSE-exposed cells (passage +29). After pSSBP2 was transfected for 24 hr, reporter constructs were transfected and incubated for further 24 hr. Renilla luciferase plasmids were cotransfected with TOPflash or FOPflash as an internal control. Each reporter activity was normalized to Renilla, and relative luciferase activity (fold) was calculated by dividing TOPflash activity by FOPflash activity. Experiments were done in four replicates and repeated twice. Values indicate means ± SD. *p < 0.05 compared to the control-Het-1A cells; #p < 0.05 compared to the empty vector-transfected MSE-Het-1A cells (t-test). (d) mRNA levels of Cyclin D1 (left) and MMP-7 (right) determined by real time-RT-PCR. (e) Treatment with Sulindac (100 μM) for 48 hr inhibited cell growth in a dose-dependent manner in both the control- and MSE-exposed cells (passage +28) and decreased Cyclin D1 and MMP-7 expression in the MSE-Het-1A cells (f). No difference was observed in TCF1 expression (data not shown). Experiments were done in four replicates and repeated twice. Values indicate means ± SD. *p < 0.05 compared to the control-Het-1A cells in the absence of Sulindac (t-test); #p < 0.05 compared to MSE-Het-1A cells in the absence of Sulindac. C, control-Het-1A cells; M, MSE-Het-1A cells. (g) TE1 and TE2 cells were treated with Sulindac (100 μM) three times a week for 2 weeks, and colony focus assays were performed. Colonies were fixed and stained with 0.4% crystal violet solution (MeOH/Acetic acid, 3:1) and taken pictures. (h) A siRNA pool targeting SSBP2 and a nontargeting control were transfected into control-Het-1A cells (C-Het-1A at the passage, +29). After 4 hr of transfection, Sulindac (100 μM) was added and incubated for a further 48 hr. Cellular growth was assessed by the MTT assay. Experiments were done in four replicates and repeated twice. Values indicate means ± SD. *p < 0.05 compared to cells transfected with control siRNA in the absence of Sulindac (t-test); #p < 0.05 compared to cells transfected with SSBP2 siRNA in the absence of Sulindac. (i) Expression of proteins involved in the Wnt signaling was examined by Western blotting in Het-1A cells (passage +32) after Sulindac treatment following transfection with control or SSBP2 siRNA. Wnt inhibitory factor-1 (WIF1) is a secreted antagonist of the Wnt pathway. β-actin was used as a loading control. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]