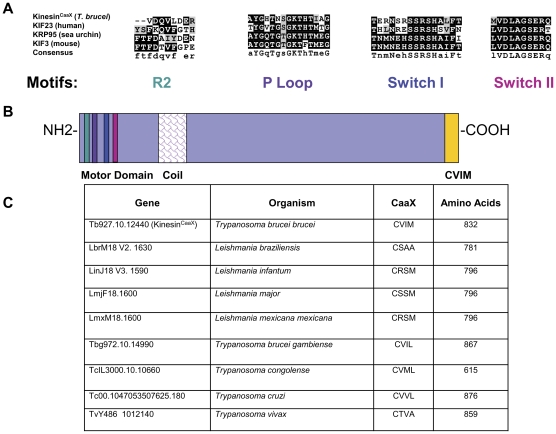
Figure 1. KinesinCaaX has motifs consistent with plus-end directed kinesin proteins and is conserved in pathogenic kinetoplastids.
(A) Key kinesin domains are conserved in Tb10.389.1270 when sequences are compared to kinesins in sea urchins, mice and humans. The corresponding GenBank accession numbers for each corresponding protein are Homo sapiens NP_612565.1, Mus musculus NP_03246, and Strongylocentrotus purpuratus P46871. (B) Structural features and domain architecture of KinesinCaaX. KinesinCaaX has 832 residues. Represented are the R2 domain (teal), the phosphate binding loop or P Loop (purple), Switch I (blue) and Switch II (magenta) regions, the coil region (fish scales) that facilitates dimerization, and CaaX motif (yellow) that is predicted to act as a farnesylation signal. (C) Comparison of KinesinCaaX orthologs in other kinetoplastids shows CaaX motif and size conservation in other pathogenic kinetoplastid species.