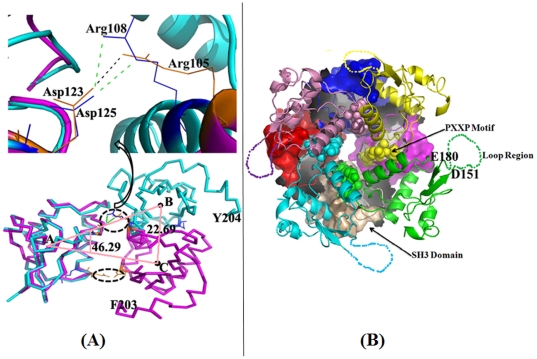
Figure 3. Dimer-tetramer oligomeric changes and occlusion of PXXP motif in HIV-1 Nef (NefRP14).
(A) (Bottom panel) Changes to the relative spatial disposition of the two chains of the Nefcore dimer (lavender) from the earlier ‘open’ structures to that observed in the present full-length tetramer (individual chains depicted in cyan). One subunit from the respective associations is superposed and the relative spatial changes to the other subunit are shown. The ‘moving’ subunit is rotated by ∼174° while the center of mass is translated by ∼22.7 Å. Despite the large changes that result in the formation of the tetramer, the interactions of Asp125 with residues of the XR motif (residues 107–108) (shown as a close-up in the upper panel) is retained. (b) One chain along with the SH3 domain (surface representation) from the Nefcore-FynSH3 complex superposed onto the tetrameric association of the full-length HIV-1Nef (cartoon representation). The SH3 domains exhibit severe steric clashes precluding their interactions with the tetrameric form of Nef. The PXXP motif (shown as spheres) cannot interact with the domain in the tetramer while the motifs present on the labeled 151–180 residue loop are exposed and can interact with other subsets of interacting protein partners.