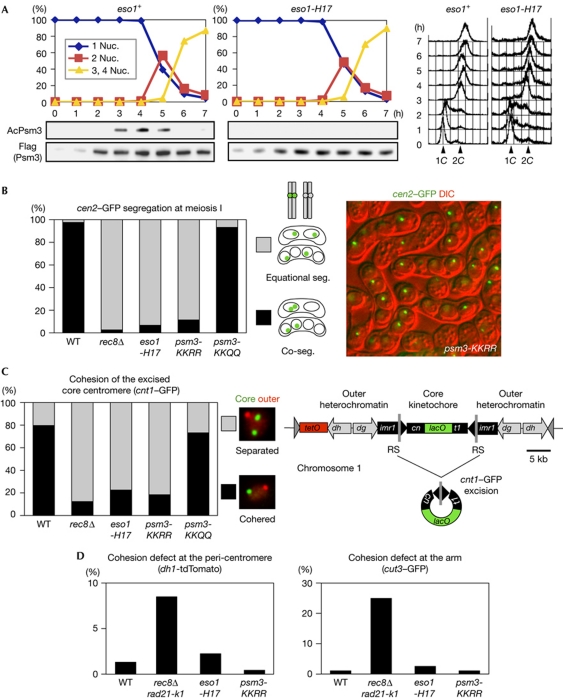
Figure 2.
Eso1-dependent acetylation of Psm3-K105/K106 is required for mono-orientation at meiosis I. (A) Haploid wild-type (WT) and eso1-H17 cells were induced to undergo synchronous meiosis by inactivating pat1-114 (Iino & Yamamoto, 1985). Cells were fixed and stained with 4,6-diamidino-2-phenylindole to monitor meiotic nuclear divisions. DNA content was measured by flow cytometry. Acetylation status of Psm3 was analysed by immunoblot. (B) Segregation (seg.) of heterozygous cen2-GFP at meiosis I was examined in the indicated rec12Δ zygotes at 26°C (n>230). Representative picture showing the cen2–GFP segregation patterns in psm3-KKRR cells. (C) Cohesion of the excised core centromere 1 in prophase I was examined in the indicated zygotes at 30°C (n>69). Schematic representation of centromere 1, in which a central core (cnt1) is surrounded by innermost repeats (imr1) and outer repeats (dg and dh), and its engineered version designed for excision (Sakuno et al, 2009). The split of cnt1 by an insertion is designated cn and t1. (D) Cohesion defect at pericentromeric and arm regions was examined in the indicated cells by arresting at prophase I (by the mei4Δ mutation) at 26°C. Strains marked with dh1-tdTomato or cut3–GFP were crossed with unmarked strains and the number of cells with two dot signals in a horsetail nucleus was counted (n>150). We used rec8Δ rad21-K1 as a cohesion defective control (Yokobayashi et al, 2003). DIC, differential interference contrast; nuc., nucleus.