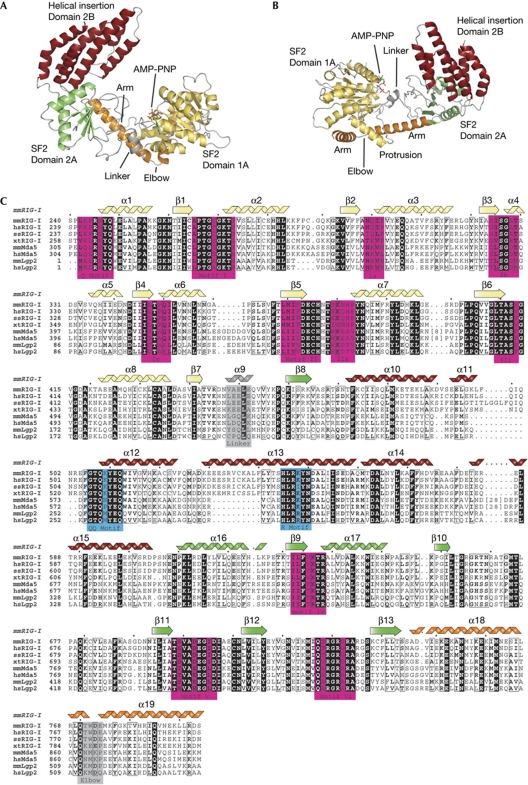
Figure 1.
Structure of the mouse RIG-I SF2 domain. (A) Front view of RIG-ISF2 along the nucleotide-binding cleft. RIG-ISF2 consists of three domains: SF2 domain 1A (yellow) and SF2 domain 2A (green) are connected by a short linker helix (grey) and form the conserved ATP-binding and hydrolysis core. The helical bundle domain 2B (red) is a specific feature of RIG-I/FANCM/Hef helicases, indicating that it is involved in double-stranded nucleic acid binding or translocation. An unusual arm (orange), unique to RIG-I-like receptors, reaches from domain 2 across domain 1 and stabilizes the observed ‘open’ conformation. (B) Top view of RIG-I, coloured as in (A). (C) Close-up view of the helical arm and its elbow (orange), embracing the helical protrusion from domain 1A, with a hydrophobic interface. (D) Structure-based sequence alignment of selected RIG-I-like receptors with highlighted conserved residues and annotated motifs. The secondary structural elements are shown on top of the alignment. AMP-PNP, adenosine 5′-(β,γ-imido)triphosphate; RIG-I, retinoic acid inducible gene I; SF2, superfamily 2.