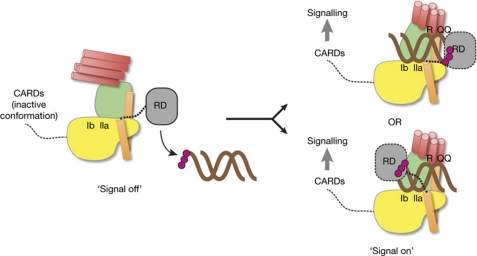
Figure 5.
Proposed model for RIG-I activation by a conformational switch in the SF2 domain. RD binds to dsRNA with 5′ triphopshates (5′-ppp; magenta spheres) and might recruit it to the SF2 domain. RNA and ATP binding switches SF2 into signal-on conformation by gripping RNA between motifs Ic/IIa on domain IA and R/QQ on domain IIB. The position of the helical arm with the short linker to RD might allow RD to bind 5′-ppp-RNA ends cooperatively with SF2. The precise position of RD, which from our structure might bind on either side of the RNA duplex (both possibilities are shown), and the position of activation and recruitment domains (CARDs) in signal-off and -on states, remain to be determined. dsRNA, double-stranded RNA; RD, repressor domain; RIG-I, retinoic acid inducible gene I; SF2, superfamily 2.