Abstract
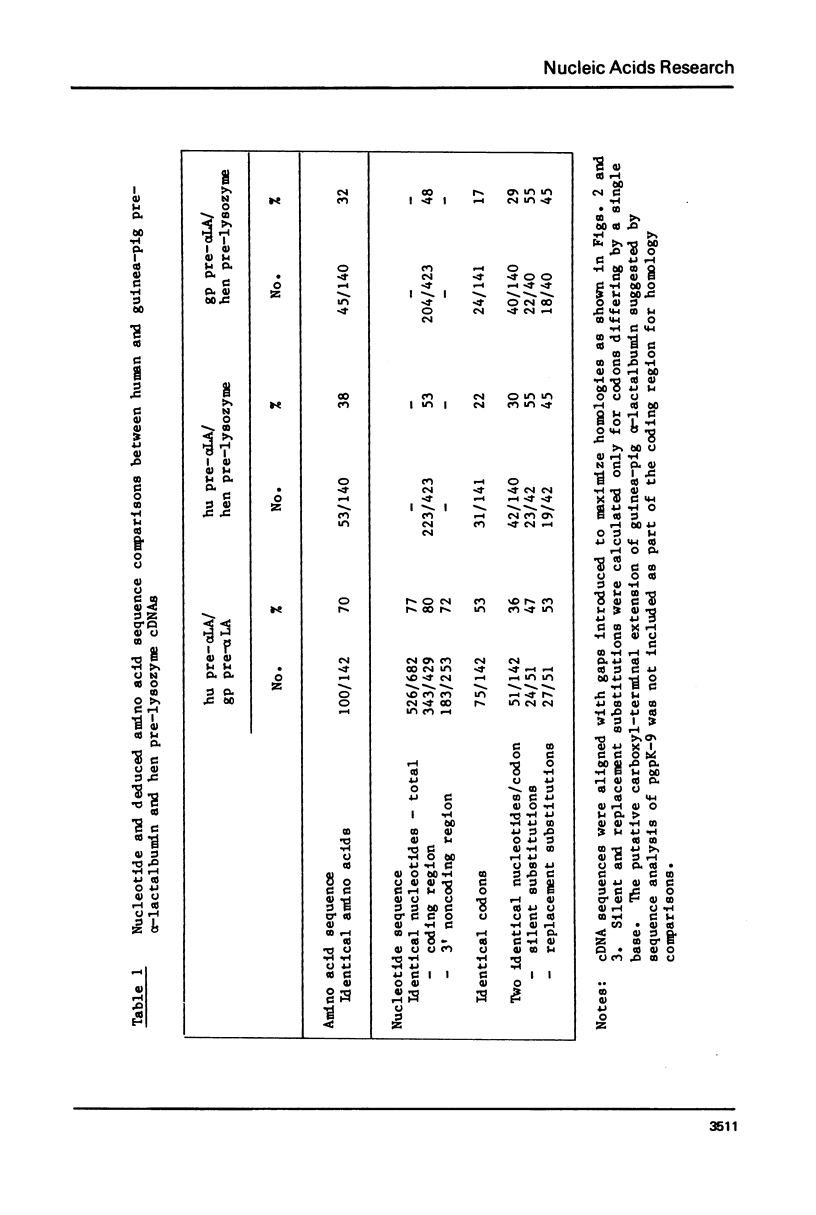
Nucleotide sequence analyses of essentially full-length copies of human and guinea-pig pre-alpha-lactalbumin cDNAs contained within recombinant plasmids, (i) confirm the presence of 19 amino acid hydrophobic amino terminal peptide extensions encoded within each mRNA; and (ii) provides evidence for the existence of a minor variant of guinea-pig alpha-lactalbumin mRNA encoding a protein with a 36 residue carboxyl-terminal extension. Comparison of the nucleotide sequence within the coding region of the human, and the predominant guinea-pig pre-alpha-lactalbumin mRNAs, with the analogous region of hen pre-lysozyme mRNA provides compelling evidence that all have evolved from a common ancestral gene.
Full text
PDF


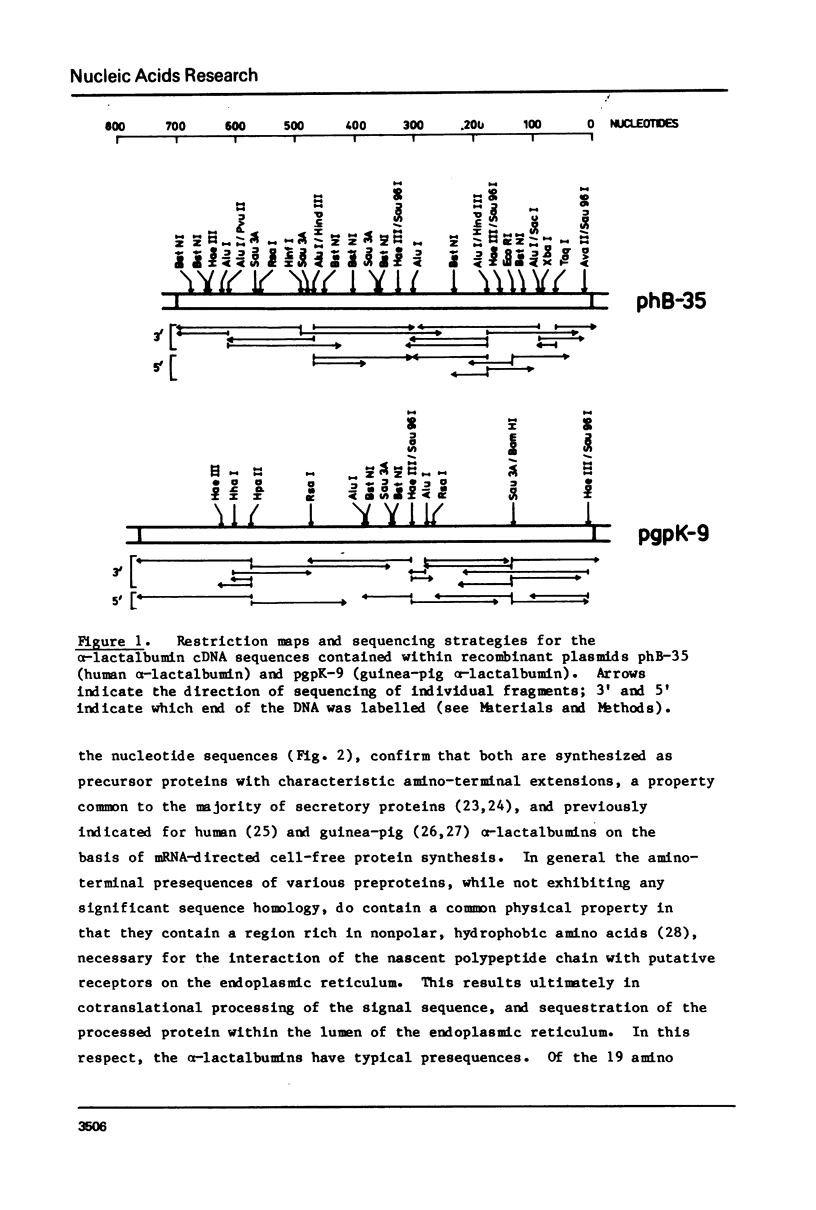








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Castellino F. J., Vanaman T. C., Hill R. L. The complete amino acid sequence of bovine alpha-lactalbumin. J Biol Chem. 1970 Sep 10;245(17):4570–4582. [PubMed] [Google Scholar]
- Brew K. The complete amino-acid sequence of guinea-pig -lactalbumin. Eur J Biochem. 1972 May 23;27(2):341–353. doi: 10.1111/j.1432-1033.1972.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. Comparison of the amino acid sequence of bovine alpha-lactalbumin and hens egg white lysozyme. J Biol Chem. 1967 Aug 25;242(16):3747–3749. [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. Resolution of a soluble lactose synthetase into two protein components and solubilization of microsomal lactose synthetase. J Biol Chem. 1966 Feb 10;241(3):762–764. [PubMed] [Google Scholar]
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- Canfield R. E., Kammerman S., Sobel J. H., Morgan F. J. Primary structure of lysozymes from man and goose. Nat New Biol. 1971 Jul 7;232(27):16–17. doi: 10.1038/newbio232016a0. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Shine J., Baxter J. D., Martial J. A. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981 Apr 25;256(8):4007–4016. [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Weiner R. I., Baxter J. D., Martial J. A. Structure of cloned DNA complementary to rat prolactin messenger RNA. J Biol Chem. 1980 Jul 10;255(13):6502–6510. [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Parker D., Campbell P. N. The construction, identification and partial characterization of plasmids containing guinea-pig milk protein complementary DNA sequences. Biochem J. 1981 Mar 15;194(3):989–998. doi: 10.1042/bj1940989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar A. M., Qasba P. K. Rat alpha-lactalbumin has a 17-residue-long COOH-terminal hydrophobic extension as judged by sequence analysis of the cDNA clones. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4853–4857. doi: 10.1073/pnas.78.8.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay J. B., Brew K. The complete amino-acid sequence of human -lactalbumin. Eur J Biochem. 1972 May;27(1):65–86. doi: 10.1111/j.1432-1033.1972.tb01812.x. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Campbell P. N. mRNA species directing synthesis of milk proteins in normal and tumour tissue from human mammary gland. Nature. 1979 Jan 4;277(5691):54–56. doi: 10.1038/277054a0. [DOI] [PubMed] [Google Scholar]
- Hall L., Davies M. S., Craig R. K. The construction, identification and characterisation of plasmids containing human alpha-lactalbumin cDNA sequences. Nucleic Acids Res. 1981 Jan 10;9(1):65–84. doi: 10.1093/nar/9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn P. H., Gait M. J., Milstein C. Complete sequence of an immunoglobulin mRNA using specific priming and the dideoxynucleotide method of RNA sequencing. Nucleic Acids Res. 1981 Sep 25;9(18):4485–4494. doi: 10.1093/nar/9.18.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Primary structure of rabbit alpha-lactalbumin. Biochemistry. 1979 Nov 13;18(23):5182–5191. doi: 10.1021/bi00590a024. [DOI] [PubMed] [Google Scholar]
- Jukes T. H., King J. L. Evolutionary nucleotide replacements in DNA. Nature. 1979 Oct 18;281(5732):605–606. doi: 10.1038/281605a0. [DOI] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray R. T., Brew K., Barnes K. The amino acid sequence of goat alpha-lactalbumin. Arch Biochem Biophys. 1979 Oct 15;197(2):404–414. doi: 10.1016/0003-9861(79)90262-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Gaye P. Study of secretory lactoproteins: primary structures of the signals and enzymatic processing. Ann N Y Acad Sci. 1980;343:232–251. doi: 10.1111/j.1749-6632.1980.tb47255.x. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T., Nishida T. Nucleotide sequence divergence and functional constraint in mRNA evolution. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7328–7332. doi: 10.1073/pnas.77.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Pascall J. C., Boulton A. P., Parker D., Hall L., Craig R. K. Heterogeneity of guinea-pig caseins synthesized and sequestered by cell-free protein-synthesizing systems. Biochem J. 1981 May 15;196(2):567–574. doi: 10.1042/bj1960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieels J. P., Bell J. E., Schindler M., Castellino F. J., Hill R. L. Involvement of histidine-32 in the biological activity of alpha-lactalbumin. Biochemistry. 1979 May 1;18(9):1771–1776. doi: 10.1021/bi00576a021. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Richardson R. H., Brew K. Lactose synthase. An investigation of the interaction site of alpha-lactalbumin for galactosyltransferase by differential kinetic labeling. J Biol Chem. 1980 Apr 25;255(8):3377–3385. [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Nussinov R., Brown R. J., Sussman J. L. Preferential codon usage in genes. Gene. 1981 May;13(4):355–364. doi: 10.1016/0378-1119(81)90015-9. [DOI] [PubMed] [Google Scholar]
- White T. J., Mross G. A., Osserman E. F., Wilson A. C. Primary structure of rat lysozyme. Biochemistry. 1977 Apr 5;16(7):1430–1436. doi: 10.1021/bi00626a030. [DOI] [PubMed] [Google Scholar]


